
����˵����ȷ���� (����)��
A����ʳ��ˮ�м���������Ũ���ᣬ���������Ե�����
B��������ͭ��Һ��̼������Һ��ϣ��õ��ij�������Cu(OH)2Ϊ����˵��������ͬ������Cu(OH)2���ܽ�ȱ�CuCO3�ĸ�С
C����0.01 mol��L��1 NaCl��Һ�м���������AgNO3��Һ���а�ɫ�������ɣ�������������Һ�м���������Ũ��ˮ����ɫ���������ܽ�
D��CaCO3��Һ�ĵ�����������������ΪCaCO3��������ʣ��������µ���ƽ�⣺CaCO3Ca2����CO32 ��
��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������������� (����)
A�������к���̼������ʴ�����ȴ�����
B���������ӵ��������������Ӵ�������
C��������Ʒ�϶�ͭʱ���Ƽ�Ϊ������ͭ��Ϊ���Һ
D����������Ƕп�飬���ܲ��ױ���ʴ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��̼���⡢��3��Ԫ����ɵ��л���A����Է�������Ϊ152���������C��Hԭ�Ӹ�����Ϊ1��1����Ԫ�ص���������Ϊ31.58%��A��FeCl3��Һ����ɫ��A����NaHCO3��Һ��Ӧ���䱽���ϵ�һ��ȡ���������֣���ش��������⣺(10��)
(1)A�ķ���ʽ��____________________________________����2�֣�
(2)A�Ľṹ��ʽΪ___________________________________����2�֣�
(3)A���Է�����ͼ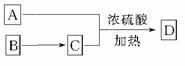 ��ʾת����ϵ��D�ķ���ʽΪC10H12O3��A��C��Ӧ�Ļ�ѧ����ʽ__________________________________��
��ʾת����ϵ��D�ķ���ʽΪC10H12O3��A��C��Ӧ�Ļ�ѧ����ʽ__________________________________��
(4)�ס�����װ�þ�������ʵ������C��ȡB��װ�ã���ͼ���ø���ԡ���� (���ͷе�290 �棬�۵�18.17��)���������¶ȴﵽ��Ӧ�¶�ʱ����ʢ��C��Ũ������Һ����ƿ��������У��ܿ�ﵽ��Ӧ�¶ȡ��ס�����װ����Ƚϣ���װ������Щ�ŵ�________����4�֣�
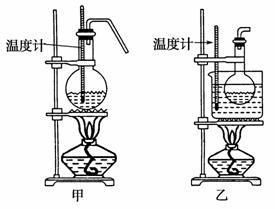
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵���У���ȷ����
A. ֻ��ȩ�����ʲ��ܷ���������Ӧ
B. ��ȩ����ȩ����ȩ��û��ͬ���칹��
C. ��2%��ϡ��ˮ��μ���2%�� ��Һ�У�������ǡ���ܽ�Ϊֹ�����Ƶ�������Һ
��Һ�У�������ǡ���ܽ�Ϊֹ�����Ƶ�������Һ
D. ��2%��NaOH��Һ4~6�Σ�����2mL10%�� ��Һ���Ƶ�
��Һ���Ƶ� ����Һ����������ȩ�����Լ�
����Һ����������ȩ�����Լ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ����(����)
A�����ܵ�������Ƚ�ʱ��KspС�ģ��ܽ��һ��С
B��Ksp��Сȡ�������ܵ���ʵ�������������Ũ�ȸı�ʱ�������ܽ�ƽ��ᷢ���ƶ�
C����ν������ȫ�����ó���������Һ��ijһ������ȫ��ȥ
D���¶�һ��������Һ��Ag����Cl��Ũ�ȵij˻�����Kspʱ����ҺΪAgCl�ı� ����Һ
����Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������±ʯ(K2SO4��MgSO4��2CaSO4��2H2O)���ڡ�������ˮ�д�������ƽ�⣺
K2SO4��MgSO4��2CaSO4��2H2O(s)2Ca2��(aq)��2K��(aq)��Mg2��(aq)��4SO42��(aq)��2H2O
Ϊ �ܳ�����ü���Դ���ñ���Ca(OH)2��Һ�ܽ���±ʯ�Ʊ�����أ���������
�ܳ�����ü���Դ���ñ���Ca(OH)2��Һ�ܽ���±ʯ�Ʊ�����أ��������� ���£�
���£�

(1)������Ҫ�ɷ���________��________�Լ�δ����±ʯ��
(2)�û�ѧƽ���ƶ�ԭ������Ca(OH)2��Һ���ܽ���±ʯ����K����ԭ��________________________________________________________________________��
(3)�����ӡ������У��ȼ���________��Һ��������Ȳ������ˣ��ټ���________��Һ����ҺpH�����ԡ�
 (4)��ͬ�¶��£�K���Ľ���Ũ�����ܽ�ʱ��Ĺ�ϵ��ͼ����ͼ�ɵã������¶����ߣ���_________��
(4)��ͬ�¶��£�K���Ľ���Ũ�����ܽ�ʱ��Ĺ�ϵ��ͼ����ͼ�ɵã������¶����ߣ���_________��
��________________________________________
________________________________________��
(5)�����Կ�����̼����Ϊ�ܽ��������ܽ������лᷢ����CaSO4(s)��CO32��CaCO3(s)��SO42��
��֪298 Kʱ��Ksp(CaCO3)��2.80��10��9��
Ksp(CaSO4 )��4.90��10��5������¶��¸÷�Ӧ��ƽ�ⳣ��K
)��4.90��10��5������¶��¸÷�Ӧ��ƽ�ⳣ��K (������������λ��Ч����)��
(������������λ��Ч����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ�������£����������ʵ�����ϵ���з�Ӧ�����Ӧ�����ӷ���ʽ��ѧ����ʽ��д��ȷ���� (����)��
A��n(Cl2)��n(Fe)��5��4
5Cl2��4Fe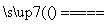 2FeCl2��2FeCl3
2FeCl2��2FeCl3
B��n(Cl2)��n(FeBr2)��1��1
Fe2����2Br����Cl2===Fe3����Br2��2Cl��
C��n(MnO4��)��n(H2O2)��2��3
2MnO4����3H2O2��6H��===2Mn2����4O2����6H2O
D��n(Fe)��n[HNO3(ϡ)]��1��3
4Fe��12H����3NO3��===3Fe2����Fe3����3NO����6H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʽC4H8O2���л�����������Һ���ȿɵ��л���A��B����A�������տɵ�C����B��CΪͬϵ���C�ɷ���������Ӧ����ԭ�л���Ľṹ��ʽΪ��(����)
A��HCOOCH2CH2CH3 ���������� B��CH3COOCH2CH3
C��CH3CH2COOCH3 ������������ D��HCOOCH��CH3��2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и��������л�Ϊͬϵ����ǣ� ��
A��CH3��CH=CH2��CH3��CH2��CH=CH2 B��CH3��CH3��CH3��CH=CH2
C��CH3��CH2��CH3��CH3��CH=CH2 D��CH3��CH2��CH2=CH2��CH3��CH2��CH3
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com