
| A£® | pH=4µÄ0.1mol/L NaHAČÜŅŗ£ŗc£ØHA-£©£¾c£ØH+£©£¾c£ØA2-£©£¾c£ØOH-£©£¾c£ØH2A£© | |
| B£® | 10mL 0.1mol/L CH3COOHČÜŅŗÓė20mL 0.1mol/L NaOHČÜŅŗ»ģŗĻŗó£¬ČÜŅŗÖŠĄė×ÓÅØ¶Č¹ŲĻµ£ŗc£ØOH-£©=c£ØH+£©+c£ØCH3COO-£©+2c£ØCH3COOH£© | |
| C£® | Į½ÖÖ“×ĖįČÜŅŗµÄĪļÖŹµÄĮæÅØ¶Č·Ö±šĪŖc1ŗĶc2£¬pH·Ö±šĪŖaŗĶa+1£¬Ōņc1£¼10c2 | |
| D£® | ŅŃÖŖ£ŗHAĪŖČõĖį£¬ŌņĮ½ÖÖČÜŅŗ¢Ł0.1mol/LHAČÜŅŗ£»¢Ś0.3mol/LHAČÜŅŗÓė0.1mol/LNaOHČÜŅŗµČĢå»żµÄ»ģŗĻŅŗ£¬c£ØH+£©¢Ł£¾¢Ś |
·ÖĪö A£®pH=4µÄ0.1mol/L NaHAČÜŅŗÖŠ£¬HA-Ąė×ÓµēĄė“óÓŚĘäĖ®½āČÜŅŗĻŌĖįŠŌ£»
B.10mL 0.1mol/L CH3COOHČÜŅŗÓė20mL 0.1mol/L NaOHČÜŅŗ»ģŗĻŗóµĆµ½“×ĖįÄĘŗĶĒāŃõ»ÆÄĘČÜŅŗ£¬ČÜŅŗÖŠ“ęŌŚµēŗÉŹŲŗćŗĶĪļĮĻŹŲŗć¼ĘĖć·ÖĪö£»
C£®“×ĖįµÄµēĄė³Ģ¶ČŗĶĖįµÄÅØ¶Č“óŠ”ÓŠ¹Ų£¬ÅضČŌ½“óµēĄė³Ģ¶ČŌ½Š”£»
D.0.1mol/LHAČÜŅŗÖŠµēĄėĘ½ŗāČÜŅŗĻŌĖįŠŌ£¬0.3mol/LHAČÜŅŗÓė0.1mol/LNaOHČÜŅŗµČĢå»żµÄ»ģŗĻµĆµ½HAŗĶNaA»ģŗĻČÜŅŗ£¬A-Ąė×Ó¶ŌHAµēĄėĘšµ½ŅÖÖĘ×÷ÓĆ£®
½ā“š ½ā£ŗA£®pH=4µÄ0.1mol/L NaHAČÜŅŗÖŠ£¬HA-Ąė×ÓµēĄė“óÓŚĘäĖ®½āČÜŅŗĻŌĖįŠŌ£¬Ąė×ÓÅضČc£ØHA-£©£¾c£ØH+£©£¾c£ØA2-£©£¾c£ØOH-£©£¾c£ØH2A£©£¬¹ŹAÕżČ·£»
B.10mL 0.1mol/L CH3COOHČÜŅŗÓė20mL 0.1mol/L NaOHČÜŅŗ»ģŗĻŗóµĆµ½“×ĖįÄĘŗĶĒāŃõ»ÆÄĘČÜŅŗ£¬ČÜŅŗÖŠ“ęŌŚµēŗÉŹŲŗćc£ØOH-£©+c£ØCH3COO-£©=c£ØH+£©+c£ØNa+£©£¬ĪļĮĻŹŲŗćĪŖ£ŗ2c£ØCH3COO-£©+2c£ØCH3COOH£©=c£ØNa+£©£¬“ųČė¼ĘĖćµĆµ½c£ØOH-£©=c£ØH+£©+c£ØCH3COO-£©+2c£ØCH3COOH£©£¬¹ŹBÕżČ·£»
C£®Čõµē½āÖŹÅضČŌ½Š”µēĄė¶ČŌ½“ó£¬pHŌ½“󣬶ųc1¦Į1=10-a£¬c2¦Į2=10-a-1£¬¦Į1£¼¦Į2£¬ĖłŅŌc1£¾10 c2£¬¹ŹC“ķĪó£»
D.0.1mol/LHAČÜŅŗÖŠµēĄėĘ½ŗāČÜŅŗĻŌĖįŠŌ£¬0.3mol/LHAČÜŅŗÓė0.1mol/LNaOHČÜŅŗµČĢå»żµÄ»ģŗĻµĆµ½HAŗĶNaA»ģŗĻČÜŅŗ£¬ČÜŅŗÖŠHAÄѶČĪŖ0.1mol/L£¬A-Ąė×Ó¶ŌHAµēĄėĘšµ½ŅÖÖĘ×÷ÓĆ£¬ĒāĄė×ÓÅØ¶Č¢Ł£¾¢Ś£¬¹ŹDÕżČ·£»
¹ŹŃ”C£®
µćĘĄ ±¾Ģāæ¼²éĮĖµē½āÖŹČÜŅŗÖŠĄė×ÓÅØ¶Č“óŠ”±Č½Ļ”¢Čõµē½āÖŹµēĄėĘ½ŗā”¢ČÜŅŗÖŠµēŗÉŹŲŗćŗĶĪļĮĻŹŲŗć¼ĘĖć·ÖĪö£¬ÕĘĪÕ»ł“”ŹĒ½āĢā¹Ų¼ü£¬ĢāÄæÄѶČÖŠµČ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ÓĆ“×Ėį³żČ„Ė®¹øÖŠµÄĢ¼ĖįøĘ£ŗCaCO3+2H+=Ca2++H2O+CO2”ü | |
| B£® | ŹÆ»ŅĖ®ÖŠ¼ÓČė¹żĮ抔ĖÕ“ņČÜŅŗ£ŗHCO3-+Ca2++OH-=CaCO3”ż+H2O | |
| C£® | ½ā±„ŗĶNaCl±„ŗĶČÜŅŗ£ŗ2Cl-+2H2O$\frac{\underline{\;Ķصē\;}}{\;}$2OH-+H2”ü+Cl2”ü | |
| D£® | ĀĮČÜÓŚĒāŃõ»ÆÄĘČÜŅŗ£ŗAl+2OH-+H2O=AlO2-+2H2”ü |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĪļÖŹµÄĮæŹĒÓĆĄ“ŃŠ¾æĪ¢¹ŪĮ£×ÓµÄŅ»øö¹ś¼Ź»ł±¾ĪļĄķĮ棬Ę䵄Ī»“¦Ä¦¶ū | |
| B£® | ĘųĢåµÄĦ¶ūĢå»żÓėĪĀ¶ČŗĶŃ¹ĒæÓŠ¹Ų£¬Ń¹ĒæŌ½“ó£¬Ģå»żŌ½“ó | |
| C£® | ČōxøöN £ØµŖ£©Ō×ÓµÄÖŹ¼¶ŹĒ1æĖ£¬Ōņ°¢·ü¼ÓµĀĀŽ³£ŹżæɱķŹ¾ĪŖ14x/mol | |
| D£® | ČĪŗĪŅ»ÖÖĪ¢¹ŪĮ£×Ó£¬µ±Ä¦¶ūÖŹĮæŅŌg/molĪŖµ„Ī»Ź±£¬Ę䏿ֵÓėÕāÖÖĮ£×ÓµÄĻą¶Ō·Ö×ÓÖŹĮæ»ņĻą¶ŌŌ×ÓÖŹĮæĻąĶ¬ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 12 g12CĖłŗ¬ÓŠµÄĢ¼Ō×ÓŹżĪŖNAøö | |
| B£® | NAµÄ½üĖĘÖµĪŖ6.02”Į1023 | |
| C£® | 2 mol H2Oŗ¬ÓŠµÄH2O·Ö×ÓŹżÄæĪŖ2NAøö | |
| D£® | 2NAøöCl2µÄÖŹĮæĪŖ71 g |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
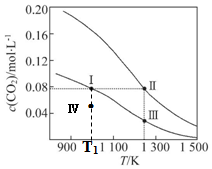
| A£® | øĆ·“Ó¦µÄ”÷H£¾0”¢”÷S£¼0 | B£® | ĢåĻµµÄ×ÜŃ¹Ēæp£ŗp £Ø¢ń£©£¾p £Ø¢ó£© | ||
| C£® | Ę½ŗā³£Źż£ŗK£Ø¢ń£©£¾K £Ø¢ņ£© | D£® | T1KŹ±£¬¢ōµćĖł“¦µÄדĢ¬ÖŠ v£ØÕż£©£¼v£ØÄę£© |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | µČĢå»ż”¢µČÅØ¶ČµÄK2CO3ČÜŅŗÓėŃĪĖį»ģŗĻ£¬Ōņ»ģŗĻŅŗÖŠ£ŗc£ØK+£©£¾c£ØCl-£©£¾c£ØHCO3-£©£¾c£ØOH-£©£¾c£ØH+£© | |
| B£® | Ļņ°±Ė®ÖŠµĪ¼ÓŃĪĖįÖĮÖŠŠŌ£¬ŌņČÜŅŗÖŠ£ŗc£ØNH4+£©=c£ØCl-£©£¾c£ØOH-£©=c£ØH+£© | |
| C£® | µČĢå»ż”¢µČÅØ¶ČµÄCH3COOHČÜŅŗÓėNaOHČÜŅŗ»ģŗĻŗó£¬ČÜŅŗµÄpH=8£¬Ōņc£ØOH-£©-c£ØCH3COOH£©=1”Į10-8mol•L-1 | |
| D£® | µČĢå»ż”¢µČÅØ¶Č¢ŁNaCl”¢¢ŚCH3COONa”¢¢ŪNaClOČÜŅŗÖŠĄė×Ó×ÜŹż“óŠ”Ė³Šņ£ŗ¢Ū£¾¢Ś£¾¢Ł |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
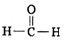 £©ÖŠŗ¬ÓŠµÄ¹²ÓƵē×Ó¶Ō×ÜŹżĪŖ2NA
£©ÖŠŗ¬ÓŠµÄ¹²ÓƵē×Ó¶Ō×ÜŹżĪŖ2NA| A£® | ¢Ł¢Ü | B£® | ¢Ū¢Ü | C£® | ¢Ś¢Ž | D£® | ¢Ś¢Ż |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
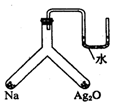 ³ä·Ö¼ÓČČČēĶ¼ĖłŹ¾µÄĆܱÕČŻĘ÷ÖŠ·ÅÖĆÓŠŹŌ¼ĮµÄĮ½øöĪ»ÖĆ£¬ČōÄĘÓėŃõ»ÆŅų¾łÓŃÓ¦ĶźĒŅ»Öø“µ½ŌĄ“µÄĪĀ¶ČŹ±£¬UŠĪ¹Ü×óÓŅĮ½²ąŅŗĆęĻąĘ½£¬ĻĀĮŠÓŠ¹ŲĖµ·Ø“ķĪóµÄŹĒ£Ø””””£©
³ä·Ö¼ÓČČČēĶ¼ĖłŹ¾µÄĆܱÕČŻĘ÷ÖŠ·ÅÖĆÓŠŹŌ¼ĮµÄĮ½øöĪ»ÖĆ£¬ČōÄĘÓėŃõ»ÆŅų¾łÓŃÓ¦ĶźĒŅ»Öø“µ½ŌĄ“µÄĪĀ¶ČŹ±£¬UŠĪ¹Ü×óÓŅĮ½²ąŅŗĆęĻąĘ½£¬ĻĀĮŠÓŠ¹ŲĖµ·Ø“ķĪóµÄŹĒ£Ø””””£©| A£® | ×°ÖĆÄŚæÕĘų³É·Ö±£³Ö²»±ä | B£® | ČČĪČ¶ØŠŌ£ŗÄʵÄŃõ»ÆĪļĒæÓŚAg2O | ||
| C£® | ×°ÖĆÄŚÄĘÓėAg2OĪļÖŹµÄĮæ±ČĪŖ2£ŗ1 | D£® | ÓŠµ»ĘÉ«¹ĢĢåÉś³É |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¶ąŃ”Ģā
| A£® | ½«pH=5µÄHCl ČÜŅŗĻ”ŹĶ1000±¶ŗópH±äĪŖ8 | |
| B£® | ½«pH=8µÄNaOHČÜŅŗĻ”ŹĶ1000±¶ŗópH±äĪŖ6 | |
| C£® | ½« pH=2µÄHCl ČÜŅŗ¼ÓČČÕō·¢£¬Ģå»ż±äĪŖŌĄ“µÄ$\frac{1}{10}$£¬pH±äĪŖ1 | |
| D£® | ½«pH=3µÄ“×ĖįČÜŅŗĻ”ŹĶ100±¶ŗó£¬pH£¼5 |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com