
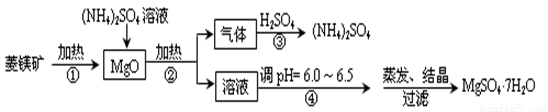
��þ���Ǽ����ͻ���ϵ���Ҫԭ�ϣ�����Ҫ�Ļ�ѧ�ɷ��ǣ�MgCO3��ͬʱ�������ʣ�SiO2��Al2O3��Fe2O3��CaO�ȣ�Ŀǰ����þ��Ϊ��Ҫԭ���Ʊ�MgSO4�ķ������£�
��֪���ٳ����£�Fe3+��Al3+��Mg2+��ʼ�γ�������������ͳ�����ȫʱ��pHֵ���£�
|
|
Fe3+ |
Al3+ |
Mg2+ |
|
��ʼ���� |
2��1 |
3��7 |
9��0 |
|
������ȫ |
3��2 |
5��2 |
12��4 |
��MgSO4��7H2O��70��80��ʱʧȥ3���ᾧˮ��300��ʱʧȥȫ���Ľᾧˮ��
��1����ƽ��ԭ�����ͣ���þ��۴��������ɵ�����þ�����ܽ����������Һ�е�ԭ�� ��
��2���ڸù�ҵ�����У�����ѭ��ʹ�õ������� ��
��3��������а���������ǡ����ȫ��Ӧ��ȡ��ʱ���õ���Һ10.00mL���250mL��Һ��������Һ�����г��ձ�������������ͷ�ιܡ���ʽ�ζ����⣬����Ҫ�IJ��������У� ������������Ƶ���ҺpH=1��c(SO42��)=1.05mol/L����������������Һ��NH4+ ˮ���ƽ�ⳣ��K��д������̣�������������λ��Ч���֣���
��4���ڼ��������������е�������������������淋����ӷ�Ӧ����ʽ�ǣ�
��
��5�������ᾧ��������Ҫʹ��60��70��ˮԡ���ȷ�ʽ����ԭ���� ��
��16�֣�
��1����NH4++H2O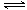 NH3��H2O+H+��MgO+ 2H+=Mg2++H2O������þ���������ӣ�笠�����ˮ��ƽ�����ҽ��У������ṩH+ʹMgO�����ܽ⣨3�֣���д����������ʽ��2�֣�ƽ�������ƶ���1�֣�
NH3��H2O+H+��MgO+ 2H+=Mg2++H2O������þ���������ӣ�笠�����ˮ��ƽ�����ҽ��У������ṩH+ʹMgO�����ܽ⣨3�֣���д����������ʽ��2�֣�ƽ�������ƶ���1�֣�
��2��(NH4)2SO4 ��2�֣�
��3��250ml����ƿ��2�֣�û�й���֣� K=5.00��10��3 mol•L��1��������̼�������
��4��Fe2O3+ 6NH4+ =6NH3��+2Fe3++3H2O(����������)��û��д������������ſ�1�֣�û��ƽ��1�֣���2�֣�
��5�������������������MgSO4��7H2Oʧȥ���ֻ�ȫ���Ľᾧˮ��2�֣�
��������
�����������1���������Һ�д�������ˮ��ƽ�⣺NH4++H2O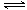 NH3•H2O+H+������þ�������ӷ�Ӧ������þ���Ӻ�ˮ����С������Ũ�ȣ��ٽ�笠����ӵ�ˮ��ƽ�����ƣ����ɵ����������ܽ���þ��۴��������ɵ�����þ����2���������������泥��������������泥�˵���ù��������У�������ǿ���ѭ�����õ����ʣ���3������250mL��Һ��Ҫ�IJ����������ձ�����������250mL����ƿ����ͷ�ιܡ���ʽ�ζ��ܵȣ����������ᷴӦ������Һ�к���H+��NH4+��OH�������ݵ���غ�ԭ���ɵã�c(H+)+c(NH4+)=2c(SO42��)������pH=1������Һ��c(H+)=0.1mol/L��c(SO42��)=1.05mol/L������c(NH4+)=2.0mol/L������(NH4)2SO4=2NH4++SO42������û��ˮ��ʱc(NH4+)=2 c(SO42��)=2.1mol/L�����������ݷ��ã�
NH3•H2O+H+������þ�������ӷ�Ӧ������þ���Ӻ�ˮ����С������Ũ�ȣ��ٽ�笠����ӵ�ˮ��ƽ�����ƣ����ɵ����������ܽ���þ��۴��������ɵ�����þ����2���������������泥��������������泥�˵���ù��������У�������ǿ���ѭ�����õ����ʣ���3������250mL��Һ��Ҫ�IJ����������ձ�����������250mL����ƿ����ͷ�ιܡ���ʽ�ζ��ܵȣ����������ᷴӦ������Һ�к���H+��NH4+��OH�������ݵ���غ�ԭ���ɵã�c(H+)+c(NH4+)=2c(SO42��)������pH=1������Һ��c(H+)=0.1mol/L��c(SO42��)=1.05mol/L������c(NH4+)=2.0mol/L������(NH4)2SO4=2NH4++SO42������û��ˮ��ʱc(NH4+)=2 c(SO42��)=2.1mol/L�����������ݷ��ã�
NH4++H2O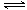 NH3•H2O+H+
NH3•H2O+H+
�������ʼŨ��/mol•L��1 2.1 0 0
����ֱ仯Ũ��/mol•L��1 0.1 0.1 0.1
�����ƽ��Ũ��/mol•L��1 2.0 0.1 0.1
K=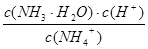 =
=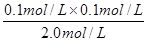 =5.00��10��3
mol•L��1��
=5.00��10��3
mol•L��1��
��4����д�����ˮ�ⷴӦ��NH4++H2O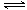 NH3��H2O+H+����дFe2O3+6H+=2Fe3++3H2O��ǰ�ߡ�6+���߿ɵã�Fe2O3+6NH4++3H2O=6NH3��H2O+2Fe3+������ʱ6NH3��H2O
NH3��H2O+H+����дFe2O3+6H+=2Fe3++3H2O��ǰ�ߡ�6+���߿ɵã�Fe2O3+6NH4++3H2O=6NH3��H2O+2Fe3+������ʱ6NH3��H2O  6NH3+6H2O����Fe2O3+6NH4+=6NH3��+2Fe3++3H2O����5�������ᾧ�õ���Ŀ�������MgSO4��7H2O�������¶ȹ��ͣ������ᾧ���ʹ��ͣ������¶ȹ��ߣ��ᾧ������MgSO4��7H2Oʧȥ���ֻ�ȫ���Ľᾧˮ����������ᾧ��������Ҫʹ��60��70��ˮԡ���ȷ�ʽ��
6NH3+6H2O����Fe2O3+6NH4+=6NH3��+2Fe3++3H2O����5�������ᾧ�õ���Ŀ�������MgSO4��7H2O�������¶ȹ��ͣ������ᾧ���ʹ��ͣ������¶ȹ��ߣ��ᾧ������MgSO4��7H2Oʧȥ���ֻ�ȫ���Ľᾧˮ����������ᾧ��������Ҫʹ��60��70��ˮԡ���ȷ�ʽ��
���㣺���������Ʊ��������̣��漰����ƽ���ƶ�ԭ������ԭ��ѭ�����õ����ʡ�����һ�������Һʹ�õIJ�����������ѧƽ�ⳣ���ļ��㡢pH�ļ��㡢�����������������Һ�����ӷ���ʽ�������ᾧ��ȡˮԡ���ȵ�ԭ����ˮ����þ���ȶ��Եȡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com