
���밴����Ҫ��д����Ӧ���ӵĵ����Ų�ʽ�����ش��й����⣺
��1��д����̬ԭ�ӻ����ӵĵ����Ų�ʽ��
Fe3+_________________��Cu _______________��
��2���Ƚ� Fe2+ ��Fe3+�Ļ�ѧ�ȶ��ԣ�Fe2+ Fe3+���뾶��С��Fe2+ Fe3+�����>����<������
��1���ڵ��������У���һ��������С��Ԫ����______���縺������Ԫ����_______����Ԫ�ط��ű�ʾ����
��2����������ԭ����p����������Ԫ����_______ ����Ԫ�ط��ű�ʾ����
��3���ڶ�����ԭ���У�δ�ɶԵ���������������������ԭ���� ����Ԫ�ط��ű�ʾ����
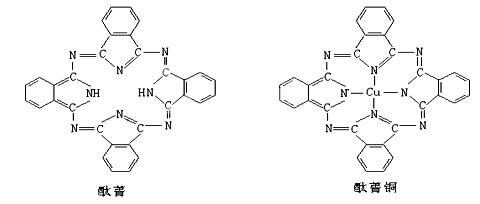
��̪ݼ��̪ݼͭȾ�Ϸ��ӽṹ����ͼ��̪ݼ�����е�ԭ�Ӳ��õ��ӻ���ʽ�У� ��������ͼ��Ӧλ���ϱ��̪ݼͭ�ṹ��ͭԭ����Χ����λ����
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�긣��ʡ�ϰ�һ�и�һ��ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ������
�밴����Ҫ��д���йط�Ӧ�����ӷ���ʽ��
��ͭ���Ȼ����ķ�Ӧ
�����Ȼ�������ͨ������
�Ǣ���10L0.1 mol��L-1��NaHCO3��Һ�е��뺬�� 0.5 mol Ba(OH)2����Һ��д����Ӧ����
�ӷ���ʽ .
����������Һ�м�������ͬŨ�ȵ�Ba(OH)2��Һ.д���˲���Ӧ�����ӷ���ʽ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�긣������̩��һ�и߶��µ�һ�νο��Ի�ѧ�Ծ����������� ���ͣ������
��.�밴����Ҫ��д����Ӧ���ӵĵ����Ų�ʽ�����ش��й����⣺
��д����̬ԭ�ӻ����ӵĵ����Ų�ʽ:
��Fe3+_________________; ��Cr _______________; ��Cu _______________.
�ƱȽ� Fe2+��Fe3+�Ļ�ѧ�ȶ��ԣ�Fe2+ Fe3+���뾶��С��Fe2+ Fe3+
����ڵ��������У���һ��������С��Ԫ����________���縺������Ԫ����________(��Ԫ�ط��ű�ʾ)��
�Ƶڶ�����������ԭ����p����������Ԫ����_______ (��Ԫ�ط��ű�ʾ)��
�ǵڶ�����ԭ����,δ�ɶԵ�������������ϵ����ԭ���� ����Ԫ�ط��ű�ʾ��
��. (2010������ģ��)ClO��ClO��ClO��Cl������sp3�ӻ������Oԭ�ӳɼ��ģ����Ʋ�������������ṹ��
| �� | ClO | ClO | ClO |
| ����ṹ | | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�������ʡĵ����һ�и߶���ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ������
���밴����Ҫ��д����Ӧ���ӵĵ����Ų�ʽ�����ش��й����⣺
��1��д����̬ԭ�ӻ����ӵĵ����Ų�ʽ��
Fe3+_________________��Cu _______________��
��2���Ƚ� Fe2+��Fe3+�Ļ�ѧ�ȶ��ԣ�Fe2+ Fe3+���뾶��С��Fe2+ Fe3+�����>����<������
��1���ڵ��������У���һ��������С��Ԫ����______���縺������Ԫ����_______����Ԫ�ط��ű�ʾ����
��2����������ԭ����p����������Ԫ����_______ ����Ԫ�ط��ű�ʾ����
��3���ڶ�����ԭ���У�δ�ɶԵ���������������������ԭ���� ����Ԫ�ط��ű�ʾ����
��̪ݼ��̪ݼͭȾ�Ϸ��ӽṹ����ͼ��̪ݼ�����е�ԭ�Ӳ��õ��ӻ���ʽ�У� ��������ͼ��Ӧλ���ϱ��̪ݼͭ�ṹ��ͭԭ����Χ����λ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�츣��ʡ��һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
�밴����Ҫ��д���йط�Ӧ�����ӷ���ʽ��
��ͭ���Ȼ����ķ�Ӧ
�����Ȼ�������ͨ������
�Ǣ���10L0.1 mol��L-1��NaHCO3��Һ�е��뺬�� 0.5 mol Ba(OH)2����Һ��д����Ӧ����
�ӷ���ʽ .
����������Һ�м�������ͬŨ�ȵ�Ba(OH)2��Һ.д���˲���Ӧ�����ӷ���ʽ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�츣������̩��һ�и߶��µ�һ�νο��Ի�ѧ�Ծ��������棩 ���ͣ������
��.�밴����Ҫ��д����Ӧ���ӵĵ����Ų�ʽ�����ش��й����⣺
��д����̬ԭ�ӻ����ӵĵ����Ų�ʽ:
��Fe3+_________________; ��Cr _______________; ��Cu _______________.
�ƱȽ� Fe2+��Fe3+�Ļ�ѧ�ȶ��ԣ�Fe2+ Fe3+���뾶��С��Fe2+ Fe3+
����ڵ��������У���һ��������С��Ԫ����________���縺������Ԫ����________(��Ԫ�ط��ű�ʾ)��
�Ƶڶ�����������ԭ����p����������Ԫ����_______ (��Ԫ�ط��ű�ʾ)��
�ǵڶ�����ԭ����,δ�ɶԵ�������������ϵ����ԭ���� ����Ԫ�ط��ű�ʾ��
��. (2010������ģ��)ClO��ClO��ClO��Cl������sp3�ӻ������Oԭ�ӳɼ��ģ����Ʋ�������������ṹ��
|
�� |
ClO |
ClO |
ClO |
|
����ṹ |
|
|
|
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com