
��֪��һ�������£�SO2��O2�������·�Ӧ��2SO2��g����O2��g��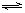 2SO3��g�� ��H��0������Ӧ�ﵽƽ����ڻ�ѧƽ�ⳣ����Kc=
2SO3��g�� ��H��0������Ӧ�ﵽƽ����ڻ�ѧƽ�ⳣ����Kc=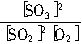 ��
��
��1�������¶ȣ��÷�Ӧ�ﵽƽ����Kcֵ_____�����������С�����䡱����ͬ�������������ת����_____����ѧ��Ӧ����_______��
��2��600��ʱ����һ�ܱ������У������������������ϣ���Ӧ������SO2��O2��SO3�����ʵ����仯����ͼ��ʾ����Ӧ����ƽ��״̬��ʱ����_______��
��3����ͼ�жϣ���Ӧ������10minʱ���߱仯��ԭ�������________����д��ţ���
A.���˴��� B.��С�������
C.�����¶� D.����SO3�����ʵ���

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �����������������ͻ�ѧ���ԣ����������������Ǹ�����ҵ����Ҫԭ�ϣ��ʻ����ᴿ�����漰��������Ӧ����Ϊ��
�����������������ͻ�ѧ���ԣ����������������Ǹ�����ҵ����Ҫԭ�ϣ��ʻ����ᴿ�����漰��������Ӧ����Ϊ��| 50�� |
| 230�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)�����CO2��H2��1��4�ı�����ϣ�ͨ�뷴Ӧ�������ʵ��������·�Ӧ���ɻ��һ����Ҫ����Դ������ɻ�ѧ����ʽ��CO2+4H2![]() _________+2H2O��
_________+2H2O��
(2)����CO2��H2��1��3�ı�����ϣ�ʹ֮������Ӧ����ij����Ҫ�Ļ���ԭ�Ϻ�ˮ�������ɵĸ���Ҫ����ԭ�Ͽ�����
A.���� B.ϩ�� C.Ȳ�� D.������
(3)��֪��443 K��473 Kʱ������(Co)��������ʹCO2��H2����C5��C8�������������˹��ϳ����͵ķ���֮һ��Ҫ�ﵽ�����͵�Ҫ��CO2��H2������ȵ�ȡֵ��Χ��V(H2)��V(CO2)��_________________________________________________________________��
(4)��֪��һ�������£��ϳ����صķ�ӦΪ��CO2(g)+2NH3(g)![]() CO(NH2)2(s)+H2O(g);��H=-127 kJ/mol���ڸ������£���44 g CO2��40 g NH3��ֻ�ϣ���Ӧ�ų�������һ��___________(����ڡ���С�ڡ����ڡ�)127 kJ������ƹ�ҵ�ϳ����ص�����___________��
CO(NH2)2(s)+H2O(g);��H=-127 kJ/mol���ڸ������£���44 g CO2��40 g NH3��ֻ�ϣ���Ӧ�ų�������һ��___________(����ڡ���С�ڡ����ڡ�)127 kJ������ƹ�ҵ�ϳ����ص�����___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����������������ͻ�ѧ���ԣ����������������Ǹ�����ҵ����Ҫԭ�ϡ��ʻ����ᴿ�����漰��������Ӧ����Ϊ��
��1��Ni(S)+4CO(g)![]()
![]() Ni(CO)4(g)+Q
Ni(CO)4(g)+Q
��2��Ni(CO)4(g) ![]() Ni(S)+4CO(g)
Ni(S)+4CO(g)
���������գ�
31.���¶Ȳ��������£�Ҫ��߷�Ӧ��1����Ni(CO4)�IJ��ʣ��ɲ�ȡ�Ĵ�ʩ�� ��
��
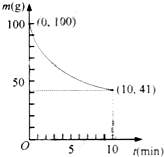
32.��֪��һ�������µ�2L�ܱ��������Ʊ�Ni(CO)4������������98.5%,�������ʲ���CO��Ӧ��ʣ�������ͷ�Ӧʱ��Ĺ�ϵ����ͼ��ʾ��Ni(CO)4��0��10min��ƽ����Ӧ����Ϊ
��
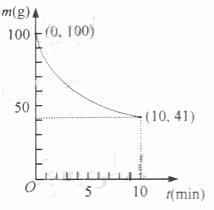
33.����Ӧ��2���ﵽƽ����������������䣬�����¶ȣ����´ﵽƽ��ʱ ��
a.ƽ�ⳣ��K���� b.CO��Ũ�ȼ�С
c.Ni��������С d.v��[Ni(CO)4]����
34.�����ʻ����ᴿ�����IJ������̡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ȫ����ͨ�ߵ�ѧУ����ͳһ���Ի�ѧ���Ϻ����������� ���ͣ������
�����������������ͻ�ѧ���ԣ����������������Ǹ�����ҵ����Ҫԭ�ϡ��ʻ����ᴿ�����漰��������Ӧ����Ϊ��
��1��Ni(S)+4CO(g)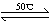 Ni(CO)4(g)+Q
Ni(CO)4(g)+Q
��2��Ni(CO)4(g) 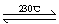 Ni(S)+4CO(g)
Ni(S)+4CO(g)
���������գ�
��1�����¶Ȳ��������£�Ҫ��߷�Ӧ��1����Ni(CO4)�IJ��ʣ��ɲ�ȡ�Ĵ�ʩ�� �� ��
��2����֪��һ�������µ�2L�ܱ��������Ʊ�Ni(CO)4������������98��5%,�������ʲ���CO��Ӧ��ʣ�������ͷ�Ӧʱ��Ĺ�ϵ����ͼ��ʾ��Ni(CO)4��0��10min��ƽ����Ӧ����Ϊ ��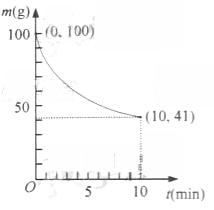
��3������Ӧ��2���ﵽƽ����������������䣬�����¶ȣ����´ﵽƽ��ʱ ��
a��ƽ�ⳣ��K���� b��CO��Ũ�ȼ�С
c��Ni��������С d��v��[Ni(CO)4]����
��4�������ʻ����ᴿ�����IJ������̡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ȫ����ͨ�ߵ�ѧУ����ͳһ���Ի�ѧ���Ϻ��������棩 ���ͣ������
�����������������ͻ�ѧ���ԣ����������������Ǹ�����ҵ����Ҫԭ�ϡ��ʻ����ᴿ�����漰��������Ӧ����Ϊ��
��1��Ni(S)+4CO(g)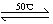 Ni(CO)4(g)+Q
Ni(CO)4(g)+Q
��2��Ni(CO)4(g) 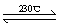 Ni(S)+4CO(g)
Ni(S)+4CO(g)
���������գ�
��1�����¶Ȳ��������£�Ҫ��߷�Ӧ��1����Ni(CO4)�IJ��ʣ��ɲ�ȡ�Ĵ�ʩ�� �� ��
��2����֪��һ�������µ�2L�ܱ��������Ʊ�Ni(CO)4������������98��5%,�������ʲ���CO��Ӧ��ʣ�������ͷ�Ӧʱ��Ĺ�ϵ����ͼ��ʾ��Ni(CO)4��0��10min��ƽ����Ӧ����Ϊ ��
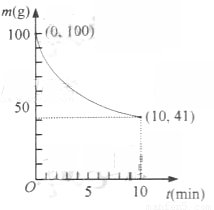
��3������Ӧ��2���ﵽƽ����������������䣬�����¶ȣ����´ﵽƽ��ʱ ��
a��ƽ�ⳣ��K���� b��CO��Ũ�ȼ�С
c��Ni��������С d��v��[Ni(CO)4]����
��4�������ʻ����ᴿ�����IJ������̡�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com