
ŹµŃéŹŅÓĆÅØĮņĖįŗĶŅŅ“¼ÖĘČ”ŅŅĻ©Ź±£¬³£»į擵½ÉÕĘæÖŠŅŗĢå±äŗŚ£¬²¢ŌŚÖʵƵÄŅŅĻ©ÖŠ»ģ
ÓŠCO2”¢SO2µČŌÓÖŹ”£Ä³æĪĶāŠ”×éÉč¼ĘĮĖČēĶ¼ĖłŹ¾×°ÖĆ£¬Ö¤Ć÷ŅŅĻ©ÖŠ»ģÓŠCO2”¢SO2²¢Ńé
Ö¤ŅŅĻ©µÄŠŌÖŹ”£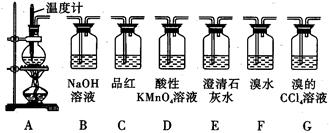
»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)ÉÕĘæÖŠĖŲÉÕ“ÉʬµÄ×÷ÓĆŹĒ ”£
(2)ČōŅŖ¼ģŃéAÖŠĖłµĆĘųĢåŗ¬ÓŠSO2£¬æɽ«»ģŗĻĘųĢåÖ±½ÓĶØČė £ØĢīĶ¼ÖŠ“śŗÅ£¬ĻĀĶ¬£©×°ÖĆ£»ČōŅŖ¼ģŃéAÖŠĖłµĆĘųĢåŗ¬ÓŠCH2=CH2£¬æɽ«»ģŗĻĘųĢåĻČĶعżB×°ÖĆŌŁĶØČė ×°ÖĆ£¬Ņ²æɽ«»ģŗĻĘųĢåøÉŌļŗóÖ±½ÓĶØČė ×°ÖĆ”£
(3)Š”Ć÷½«“ÓA³öĄ“µÄ»ģŗĻĘųĢåŅĄ“ĪĶعżB”¢E”¢D”¢E£¬·¢ĻÖDĒ°ĆęµÄŹÆ»ŅĖ®ÖŠĪŽĆ÷ĻŌ±ä»Æ”¢DŗóĆęµÄŹÆ»ŅĖ®±ä»ė×Ē”£Ēė¶Ō³öĻÖøĆĻÖĻóµÄŌŅņ½ųŠŠŗĻĄķ²ĀĻė ”£
(4)ČōŅŖŅ»“ĪŠŌ½«ČżÖÖĘųĢåČ«²æ¼ģŃé³öĄ“£ŗ
¢Ł¼ģŃéµÄĖ³ŠņŹĒ£ØĢīĘųĢåĆū³Ę£© £»
¢ŚÉĻŹö×°ÖĆ×é×°µÄĖ³ŠņÖŠ×ī¼ņ±ćµÄŹĒ£ŗA”ś ,Ö¤Ć÷C02“ęŌŚµÄĻÖĻóŹĒ ”£
£Ø1£©·ĄÖ¹±©·Š
£Ø2£© C D(»ņF»ņG) G
£Ø3£© ŅŅĻ©±»ĖįŠŌøßĆĢĖį¼ŲČÜŅŗŃõ»Æ³ÉCO2
(4) ¢ŁŅŅĻ©”¢¶žŃõ»ÆĮņ”¢¶žŃõ»ÆĢ¼
¢ŚGCFE FÖŠäåĖ®²»ĶŹÉ«£¬EÖŠ³ĪĒåŹÆ»ŅĖ®±ä»ė×Ē
½āĪöŹŌĢā·ÖĪö£ŗ(1)ÖĘČ”ŅŅĻ©ŹĒÓĆÅØĮņĖįŗĶŅŅ“¼µÄ»ģŗĻŅŗŌŚ170”ęµÄĪĀ¶ČĻĀÖĘµĆ£¬ÓÉÓŚ²ā¶ØµÄŹĒ»ģŗĻŅŗµÄĪĀ¶Č£¬ĖłŅŌĪĀ¶Č¼Ę±ŲŠė²åČėŅŗĆęŅŌĻĀ£¬ÓÉ“ĖĄ“æ“·“Ó¦ĪļÖ»ÓŠŅŗĢ壬Ņņ“ĖŠčŅŖ¼ÓČėĖé“ÉʬµČ¹ĢĢ壬·ĄÖ¹ŅŗĢ屩·Š”£¹Ź“š°øĪŖ£ŗ·ĄÖ¹±©·Š
øł¾ŻCO2”¢SO2ÓėŅŅĻ©ČÜŅŗ·“Ó¦£¬O2ÓėŅŅĻ©¶¼ÄÜÓėäåĖ®”¢ĖįŠŌøßĆĢĖį¼ŲČÜŅŗ·“Ó¦£¬µ«ŹĒÖ»ÓŠSO2ÄÜÓėĘ·ŗģČÜŅŗ·“Ó¦£¬Ö»ÓŠŅŅĻ©ÄÜÓėäåµÄĖÄĀČ»ÆĢ¼ČÜŅŗ·“Ó¦£¬ĖłŅŌ¼ģŃéCO2Ē°±ŲŠė¼ģŃé²¢³żČ„SO2£¬¼ģŃéSO2Ē°±ŲŠė¼ģŃé²¢³żČ„ŅŅĻ©”£¹Ź“š°øĪŖ£ŗC D(»ņF»ņG) G
Ź¹Ö®ŗóµÄŹÆ»ŅĖ®±ä»ė×ĒµÄÖ»ÓŠCO2£¬µ«ŹĒDÖ®Ē°µÄŹÆ»ŅĖ®ĪŽĻÖĻó£¬ĖµĆ÷CO2²»ŹĒŌĄ“¾ĶÓŠµÄ£¬Ö»ÄÜŹĒŠĀÉś³ÉµÄ£¬Ņ²Ö»ÄÜŹĒŅŅĻ©Ńõ»ÆŗóµÄ²śĪļ”£¹Ź“š°øĪŖ£ŗŅŅĻ©±»ĖįŠŌøßĆĢĖį¼ŲČÜŅŗŃõ»Æ³ÉCO2
£Ø4£©¢ŁŅŅĻ©”¢¶žŃõ»ÆĮņ”¢¶žŃõ»ÆĢ¼
¢Ś×°ÖĆ×é×°µÄĖ³ŠņÖŠ×ī¼ņ±ćµÄŹĒGCFE £»ŅŖ¼ģŃé C02ŹĒ·ń“ęŌŚĻČŅŖ¼ģŃéŅŅĻ©ŹĒ·ń“ęŌŚ£¬ŌņŠčĶØČėäåĖ®Čē¹ūFÖŠäåĖ®²»ĶŹÉ«£¬EÖŠ³ĪĒåŹÆ»ŅĖ®±ä»ė×Ē£¬ŌņC02“ęŌŚ”£¹Ź“š°øĪŖ£ŗFÖŠäåĖ®²»ĶŹÉ«£¬EÖŠ³ĪĒåŹÆ»ŅĖ®±ä»ė×Ē
æ¼µć£ŗ±¾Ģā×ÅÖŲæ¼²éÓĆÅØĮņĖįŗĶŅŅ“¼ÖĘČ”ŅŅĻ©µÄŹµŃ锣



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
ŃĒĻõĖįÄʱ»³ĘĪŖ¹¤ŅµŃĪ£¬ŌŚĘÆ°×”¢µē¶ĘµČ·½ĆęÓ¦ÓĆ¹ć·ŗ”£ŅŌľĢ攢ÅØĻõĖį”¢Ė®ŗĶĶĪŖŌĮĻÉś³ÉµÄŅ»Ńõ»ÆµŖÓė¹żŃõ»ÆÄĘ·“Ó¦ÖʱøŃĒĻõĖįÄʵÄ×°ÖĆČēĻĀĶ¼ĖłŹ¾£Ø²æ·Ö¼Š³Ö×°ÖĆĀŌ£©”£
ŅŃÖŖ£ŗŹŅĪĀĻĀ£¬¢Ł2NO+Na2O2”ś2NaNO2 ¢Ś3NaNO2+3HCl”ś3NaCl+HNO3+2NO”ü+H2O£»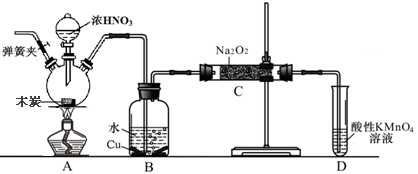
¢ŪĖįŠŌĢõ¼žĻĀ£¬NO»ņNO2ØC¶¼ÄÜÓėMnO4ØC·“Ӧɜ³ÉNO3ØCŗĶMn2+
Ķź³ÉĻĀĮŠĢīæÕ£ŗ
£Ø1£©Š“³öÅØĻõĖįÓėľĢæ·“Ó¦µÄ»Æѧ·½³ĢŹ½ ”£
£Ø2£©BÖŠ¹Ū²ģµ½µÄÖ÷ŅŖĻÖĻóŹĒ £¬D×°ÖƵÄ×÷ÓĆŹĒ ”£
£Ø3£©¼ģŃéCÖŠ²śĪļÓŠŃĒĻõĖįÄʵķ½·ØŹĒ ”£
£Ø4£©¾¼ģŃéC²śĪļÖŠ³żŃĒĻõĖįÄĘĶā»¹ŗ¬ÓŠø±²śĪļĢ¼ĖįÄĘŗĶ £¬ĪŖ±ÜĆā²śÉśÕāŠ©ø±²śĪļÓ¦ŌŚB”¢C×°ÖĆ¼äŌö¼Ó×°ÖĆE£¬»³öE²¢×¢Ć÷EÖŠŹ¢·ÅµÄŅ©Ę·Ćū³Ę ”£
£Ø5£©Š“³ö¼ģŃéC²śĪļÖŠŹĒ·ńŗ¬Ģ¼ĖįÄʵķ½·Ø ”£
£Ø6£©½«1.56g¹żŃõ»ÆÄĘĶźČ«×Ŗ»Æ³ÉĪŖŃĒĻõĖįÄĘ£¬ĄķĀŪÉĻÖĮÉŁŠčŅŖľĢæ g”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
¢ńij»Æѧ»ī¶ÆŠ”×éÉč¼ĘČēĻĀĶ¼ĖłŹ¾£Ø²æ·Ö¼Š³Ö×°ÖĆŅŃĀŌČ„£©ŹµŃé×°ÖĆ£¬ŅŌĢ½¾æ³±ŹŖµÄCl2ÓėøÉŌļNa2CO3 ¹ĢĢå·“Ó¦µĆµ½µÄ¹ĢĢåĪļÖŹµÄ³É·Ö”£
ŅŃÖŖ£ŗĶØČėŅ»¶ØĮæµÄĀČĘųŗ󣬲āµĆDÖŠÖ»ÓŠŅ»ÖÖ³£ĪĀĻĀĪŖ»ĘŗģÉ«µÄĘųĢ壬ĘäĪŖŗ¬ĀČŃõ»ÆĪļ”£æÉŅŌČ·¶ØµÄŹĒCÖŠ¹ĢĢåŗ¬ÓŠNaHCO3 £¬ĒŅŗ¬ĀȵÄŃĪÖ»ÓŠŅ»ÖÖ”£ĻÖ¶ŌCµÄ³É·Ö½ųŠŠ²ĀĻėŗĶĢ½¾æ”£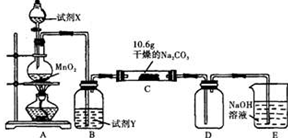
£Ø1£©Ģį³öŗĻĄķ²ĀĻė£ŗČōŅŃÖŖCÖŠÓŠ0.1molCl2Ē”ŗĆŗĶ10.6æĖNa2CO3¹ĢĢåĶźČ«·“Ó¦”£ŌņCÖŠÓŠCl2²ĪÓė·“Ó¦µÄ»Æѧ·½³ĢŹ½æÉÄÜ ”£
£Ø2£©¢ŁĢį³öŗĻĄķ¼ŁÉč”£
¼ŁÉč1£ŗ“ęŌŚĮ½Öֳɷ֣ŗNaHCO3ŗĶ £»
¼ŁÉč2£ŗ“ęŌŚČżÖֳɷ֣ŗNaHCO3ŗĶ ”¢ ”£
¢ŚÉč¼ĘŗĻĄķ·½°ø¶ŌC¹ĢĢåÖŠµÄĪ“ÖŖ³É·Ö½ųŠŠĢ½¾æ”£ĒėŠ“³öŹµŃé²½ÖčŅŌ¼°Ō¤ĘŚĻÖĻóŗĶ½įĀŪ£Øæɲ»ĢīĀś£©”£
ĻŽŃ”ŹµŃéŹŌ¼ĮŗĶŅĒĘ÷£ŗÕōĮóĖ®”¢Ļ”HNO3”¢Ba(OH)2ČÜŅŗ”¢BaCl2ČÜŅŗ”¢³ĪĒåŹÆ»ŅĖ®”¢AgNO3ČÜŅŗ”¢ŹŌ¹Ü”¢Š”ÉÕ±”£
| ŹµŃé²½Öč | Ō¤ĘŚĻÖĻóŗĶ½įĀŪ |
| ²½Öč1£ŗȔɣĮæCÖŠ¹ĢĢåѳʷӌŹŌ¹ÜÖŠ£¬¼ÓČė×ćĮæÕōĮóĖ®£¬³ä·ÖÕńµ“ÖĮ¹ĢĢåĶźČ«Čܽā£¬Č»ŗó½«ĖłµĆČÜŅŗ·Ö×°A”¢BĮ½Ö§ŹŌ¹ÜÖŠ”£ | |
| ²½Öč2£ŗ | |
| ²½Öč3£ŗ | |
| | |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
ŗ£Ė®ŹĒ¾Ž“óµÄ׏Ō“±¦æā£¬“Óŗ£Ė®ÖŠĢįČ”Ź³ŃĪŗĶäåµÄ¹ż³ĢČēĻĀ£ŗ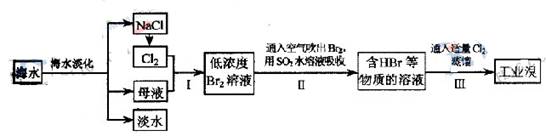
£Ø1£©²½Öč¢ńÖŠŅŃ»ńµĆBr2£¬²½Öč¢ņÖŠÓÖ½«Br2»¹ŌĪŖBr?£¬ĘäÄæµÄĪŖ ”£
£Ø2£©²½Öč¢ņÓĆSO2Ė®ČÜŅŗĪüŹÕBr2£¬ĪüŹÕĀŹæÉ“ļ95£„£¬ÓŠ¹Ų·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ £¬ÓÉ“Ė·“Ó¦æÉÖŖ£¬³ż»·¾³±£»¤Ķā£¬ŌŚ¹¤ŅµÉś²śÖŠÓ¦½ā¾öµÄÖ÷ŅŖĪŹĢāŹĒ ”£
£Ø3£©Ä³»Æѧъ¾æŠŌѧĻ°Š”×éĪŖĮĖ½ā“Ó¹¤ŅµäåÖŠĢį“æäåµÄ·½·Ø£¬²éŌÄĮĖÓŠ¹Ų׏ĮĻ£¬Br2µÄ·ŠµćĪŖ59”ę”£Ī¢ČÜÓŚĖ®£¬ÓŠ¶¾ŠŌŗĶĒæøÆŹ“ŠŌ”£ĖūĆĒ²Ī¹ŪÉś²ś¹ż³Ģŗó£¬ĮĖČēĻĀ×°ÖĆ¼ņĶ¼£ŗ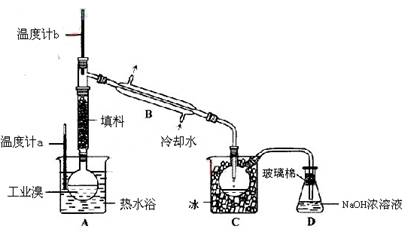
ĒėÄć²ĪÓė·ÖĪöĢÖĀŪ£ŗ
¢ŁĶ¼ÖŠŅĒĘ÷BµÄĆū³Ę ”£
¢ŚÕūĢ׏µŃé×°ÖĆÖŠŅĒĘ÷Į¬½Ó¾ł²»ÄÜÓĆĻš½ŗČūŗĶĻš½ŗ¹Ü£¬ĘäŌŅņŹĒ ”£
¢ŪŹµŃé×°ÖĆĘųĆÜŠŌĮ¼ŗĆ£¬ŅŖ“ļµ½Ģį“æäåµÄÄæµÄ£¬²Ł×÷ÖŠČēŗĪæŲÖĘ¹Ų¼üĢõ¼ž£ŗ ”£
¢ÜCÖŠŅŗĢå²śÉśŃÕÉ«ĪŖ ”£ĪŖ³żČ„øĆ²śĪļÖŠČŌ²ŠĮōµÄÉŁĮæCl2£¬æÉĻņĘäÖŠ¼ÓČėNaBrČÜŅŗ£¬³ä·Ö·“Ó¦ŗó£¬ŌŁ½ųŠŠµÄ·ÖĄė²Ł×÷ŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
ŗ£ŃóÉśĪļČēŗ£“ų”¢ŗ£ŌåÖŠŗ¬ÓŠ·įø»µÄµāŌŖĖŲ£¬Ö÷ŅŖŅŌµā»ÆĪļŠĪŹ½“ęŌŚ”£ÓŠŅ»»ÆѧæĪĶāŠ”×éÓĆŗ£“ųĪŖŌĮĻÖĘȔɣĮæµāµ„ÖŹ£¬ĖūĆĒ½«ŗ£“ų×ĘÉճɻŅ£¬ÓĆĖ®½žÅŻŅ»¶ĪŹ±¼ä(ŅŌČƵā»ÆĪļ³ä·ÖČܽāŌŚĖ®ÖŠ)£¬µĆµ½ŗ£“ų»ŅŠü×ĒŅŗ£¬Č»ŗó°“ŅŌĻĀŹµŃéĮ÷³ĢĢįČ”µ„ÖŹµā£ŗ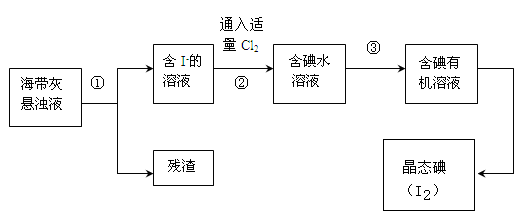
£Ø1£©Öø³öĢįČ”µāµÄ¹ż³ĢÖŠÓŠ¹ŲµÄŹµŃé²Ł×÷Ćū³Ę£ŗ¢Ł_________£¬¢Ū________”£
£Ø2£©¹ż³Ģ¢ŚÖŠ³äČėŹŹĮæCl2·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ_____________________”£
£Ø3£©²Ł×÷¢ŪÖŠĖłÓƵÄÓŠ»śŹŌ¼ĮæÉŅŌŹĒ___________(Ö»ĢīŅ»ÖÖ)£¬¼ņŹöŃ”ŌńĘäĄķÓÉ__________________”£
£Ø4£©“Óŗ¬µāµÄÓŠ»śČÜŅŗÖŠĢįČ”µāŗĶ»ŲŹÕÓŠ»śČÜŅŗ£¬»¹ŠčŅŖ¾¹żÕōĮ󣬹Ū²ģĻĀĶ¼ĖłŹ¾ŹµŃé×°ÖĆ£¬Öø³öĘä“ķĪóÖ®“¦²¢¼ÓŅŌøÄÕż_______________________________________________________”£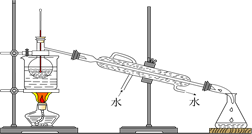
(5)½ųŠŠÉĻŹöÕōĮó²Ł×÷Ź±£¬Ź¹ÓĆĖ®Ō”µÄŌŅņŹĒ______________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
ij»ÆѧŠĖȤŠ”×éµÄĶ¬Ń§ĶعżŅŌĻĀ²½ÖčĢįČ”ŗ£“ųÖŠµÄµā£ŗ
¢Ł½«ŗ£“ųɹøÉ×ĘÉճɻŅ ¢Ś½«ŗ£“ų»Ņ½žÅŻµĆĘäŠü×ĒŅŗ ¢Ū¹żĀĖµĆŗ¬µāĄė×ÓµÄČÜŅŗ ¢ÜĻņøĆČÜŅŗÖŠ¼ÓČėŹŹĮæCl2ÖĆ»»³öµā ¢Ż¶Ōŗ¬µāČÜŅŗ½ųŠŠŅ»ĻµĮŠµÄĢįČ”ŗóµĆµāµ„ÖŹ
£Ø1£©×ĘÉÕ¹ż³ĢæÉŌŚĻĀĮŠÄĒÖÖŅĒĘ÷ÖŠ½ųŠŠ_____________
| A£®ÉÕ± | B£®ŹŌ¹Ü | C£®Õō·¢Ćó | D£®ŪįŪö |

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
ŹµŃéŹŅÖʱø°±»ł¼×Ėįļ§£ØNH2COONH4£©µÄ·“Ó¦ČēĻĀ£ŗ2NH3(g)£«CO2(g)  NH2COONH4(s)£¬øĆ·“Ó¦ŌŚøÉŌļĢõ¼žĻĀ½öÉś³É°±»ł¼×Ėįļ§£¬ČōÓŠĖ®“ęŌŚŌņÉś³ÉĢ¼Ėįļ§»ņĢ¼ĖįĒāļ§”£
NH2COONH4(s)£¬øĆ·“Ó¦ŌŚøÉŌļĢõ¼žĻĀ½öÉś³É°±»ł¼×Ėįļ§£¬ČōÓŠĖ®“ęŌŚŌņÉś³ÉĢ¼Ėįļ§»ņĢ¼ĖįĒāļ§”£
£Ø1£©øĆ·“Ó¦ŌŚŅ»¶ØĢõ¼žĻĀÄܹ»×Ō·¢½ųŠŠ£¬Ōņ·“Ó¦µÄ¦¤H 0”££ØĢī“óÓŚ”¢Š”ÓŚ»ņµČÓŚ£©
£Ø2£©Š“³öÉś³ÉĢ¼ĖįĒāļ§µÄ»Æѧ·½³ĢŹ½ ”£
£Ø3£©°“ĻĀĶ¼×°ÖĆ½ųŠŠČēĻĀŹµŃé£ŗ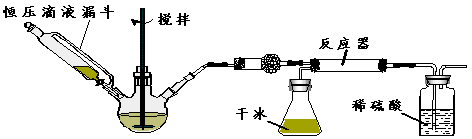
²½Öč1£ŗ¼ģ²é×°ÖĆĘųĆÜŠŌ”£
²½Öč2£ŗŌŚĻąÓ¦ŅĒĘ÷֊װČėŅ©Ę·£¬ĘäÖŠŌŚČż¾±ÉÕĘæÖŠ¼ÓČė×ćĮæµÄĒāŃõ»ÆÄĘ¹ĢĢ壬ŗćŃ¹µĪŅŗĀ©¶·ÖŠ×°ČėÅØ°±Ė®”£
²½Öč3£ŗµĪ¼ÓÅØ°±Ė®²¢½Į°č£¬µ÷½Ś·“Ó¦ĖŁĀŹ£¬ŌŚ·“Ó¦Ę÷ÖŠµĆµ½²śĘ·
””
¢ŁøÉŌļ¹ÜÖŠŹ¢·ÅµÄŅ©Ę·ŹĒ ”£
¢Ś¶Ō±ČĢ¼ĖįŃĪŗĶĖį·“Ó¦ÖĘCO2£¬øĆŹµŃéĄūÓĆøɱłÉż»Ŗ²śÉśCO2ĘųĢåµÄÓŵćÓŠ ”£
¢ŪŅŌŗćŃ¹µĪŅŗĀ©¶·“śĢę·ÖŅŗĀ©¶·µÄÄæµÄŹĒ ”£
¢Ü·“Ó¦ŗóĘŚĖę×ÅCO2ĘųĮ÷¼õĀż£¬µ¼ÖĀ·“Ó¦Īļ±ČĄż²»µ±£¬æɲÉČ”µÄĻąÓ¦“ėŹ©ŹĒ ”£
£Ø4£©ÓŠĶ¬Ń§ČĻĪŖøĆŹµŃé×°ÖĆ“ęŌŚ°²Č«ĪŹĢā£¬ĒėĪŹæÉÄÜĆęĮŁµÄ°²Č«ĪŹĢāŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
I”¢Ä³»ÆѧæĪĶā»ī¶ÆŠ”×é¶ŌĪŪČ¾“óĘųµÄ²æ·Ö·Ē½šŹōŃõ»ÆĪļ½ųŠŠĢ½¾æ”£Ēėøł¾ŻĢāÄæŅŖĒó»Ų“šĻĀĮŠĪŹĢā”£
(1)Š“³öÓĆĻõĖįÖĘČ”NOµÄĄė×Ó·½³ĢŹ½ ”£
(2)²é׏ĮĻµĆÖŖ£¬HCOOH CO+H2O”£ŹµŃéŹŅÓŠČēĶ¼lĖłŹ¾µÄ×°ÖĆ£¬ÖĘČ”COĘųĢåæÉŃ”ÓƵÄ×°ÖĆĪŖ
CO+H2O”£ŹµŃéŹŅÓŠČēĶ¼lĖłŹ¾µÄ×°ÖĆ£¬ÖĘČ”COĘųĢåæÉŃ”ÓƵÄ×°ÖĆĪŖ
(ĢīŠņŗÅ)£¬ŹµŃéŹŅĄūÓĆøĆ×°ÖĆ»¹æÉÖĘČ”µÄ³£¼ūĘųĢåÓŠ £ØŠ“Ņ»ÖÖĘųĢåµÄ·Ö×ÓŹ½£©”£
(3)²é׏ĮĻµĆÖŖ£¬ĄūÓĆ“ß»Æ¼ĮæÉŹ¹Ęū³µĪ²ĘųÖŠµÄŅ»Ńõ»ÆĢ¼ŗĶµŖŃõ»ÆĪļ“ó²æ·Ö·¢Éś·“Ó¦×Ŗ»ÆĪŖ¶žŃõ»ÆĢ¼ŗĶµŖĘų”£øĆŠ”×éŌŚŹµŃéŹŅÄ£ÄāĘū³µĪ²Ęų“¦Ąķ£¬Éč¼ĘĮĖČēĶ¼2ĖłŹ¾×°ÖĆ(²æ·Ö¼Š³ÖŗĶ×°ÖĆŅŃĀŌČ„)”£

¢ŁŹµŃéĒ°£¬¹Ų±ÕŠżČūK£¬ĻČĶصŖĘųÅž»×°ÖĆÖŠµÄæÕĘų£¬ĘäÄæµÄŹĒ ”£
¢Ś×°ÖĆ(III)µÄÖ÷ŅŖ×÷ÓĆŹĒ ”£
¢ŪøĆĢ××°ÖĆÖŠÓŠ²»ĶźÉĘÖ®“¦£¬»¹Ó¦ŌŚ×°ÖĆ(¢ō)ŗó²¹³ä ×°ÖĆ”£
II”¢øĆæĪĶāŠ”×éÉč¼ĘµÄ“ÓZnSO4”¢FeCl3µÄ»ģŗĻČÜŅŗÖŠÖĘČ”ZnSO4”¤7H2O¹ż³ĢČēĻĀ£ŗ
a£®ŌŚ»ģŗĻŅŗÖŠ¼ÓČė6 mol/L NaOHČÜŅŗ£¬ÖĮpH=8ĪŖÖ¹”£
b£®¹żĀĖŗóµĆµ½³Įµķ£¬ÓĆÕōĮóĖ®¶ą“ĪĻ“µÓ³Įµķ”£
c£®ĻņĻ“µÓŗóµÄ³ĮµķÖŠ¼ÓČė2 mol/LµÄĮņĖį£¬±£³ÖČÜŅŗµÄpHŌŚ4”«6£¬¼ÓČČÖó·Š£¬³ĆČČ¹żĀĖ£¬ĀĖŅŗ¼“ĪŖZnSO4ČÜŅŗ”£
d£®ĀĖŅŗÖŠ¼ÓČė2 mol/LµÄĮņĖį£¬Ź¹ĘäpH=2”£
ŅŃÖŖ²æ·ÖŃōĄė×ÓŅŌĒāŃõ»ÆĪļµÄŠĪŹ½æŖŹ¼³ĮµķÖĮĶźČ«³ĮµķŹ±ČÜŅŗµÄpH¼ūĻĀ±ķ£¬»Ų“šĻĀĮŠĪŹĢā£ŗ
| ³ĮµķĪļ | Fe(OH)3 | Zn(OH)2 |
| pH | 1.5”«3.2 | 6.4”«8.0 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
µāŌŖĖŲÓŠ”°ÖĒĮ¦ŌŖĖŲ”±Ö®³Ę”£ŃŠ¾æŠŌѧĻ°Š”×é×öĮĖČēĻĀŹµŃéĢ½¾æŗ£“ųÖŠµāŌŖĖŲ“ęŌŚ²¢²ā¶ØĘäÖŠµāŌŖĖŲµÄŗ¬Į攣
£Ø1£©²Ł×÷IĪŖ×ĘÉÕ£¬Ōņ×ĘÉÕŹ±ÓĆ____Ź¢×°ŗ£“ų£¬²Ł×÷IIĪŖ____________________________£»
£Ø2£©Ė®½žŹ±Ķس£ŅŖ½«Šü×ĒŅŗÖó·Š2”«3min£¬ÄæµÄŹĒ______________________________£»
£Ø3£©²Ł×÷III£¬ŹĒĶ¬Ń§ĆĒ¶ŌČÜŅŗAÖŠµāŌŖĖŲµÄ“ęŌŚŠĪŹ½½ųŠŠµÄĢ½¾æŹµŃ锣
[ĶĘ²ā]£ŗ¢ŁŅŌIO3”„ŠĪŹ½“ęŌŚ£» ¢ŚŅŌI”„ŠĪŹ½“ęŌŚ
[²éŌÄ׏ĮĻ]£ŗIO3”„¾ßÓŠ½ĻĒæµÄŃõ»ÆŠŌ£¬I2+2S2O32”„=2I”„+S4O62”„
½«ÉĻŹöČÜŅŗĻ”ŹĶÅäÖĘ³É200mLČÜŅŗ£¬ĒėĶź³ÉĻĀĮŠŹµŃéĢ½¾æ”£ĻŽŃ”ŹŌ¼Į£ŗ3%H2O2ČÜŅŗ”¢KSCNČÜŅŗ”¢FeCl2ČÜŅŗ”¢Ļ”ĮņĖį”£
| ŠņŗÅ | ŹµŃé·½°ø | ŹµŃéĻÖĻó | ½įĀŪ |
| ¢Ł | ȔɣĮæĻ”ŹĶŗóµÄČÜŅŗA¼ÓČėµķ·ŪŗóŌŁÓĆĮņĖįĖį»Æ£¬·Ö×°ÓŚŹŌ¹ÜI”¢II | ĪŽĻÖĻó | |
| ¢Ś | ĶłŹŌ¹ÜIÖŠ¼ÓČė______ | ĪŽĻÖĻó | Ö¤Ć÷²»ŹĒŅŌIO3”„ŠĪŹ½“ęŌŚ |
| ¢Ū | ĶłŹŌ¹ÜIIÖŠ¼ÓČė_______ | _______________ | Ö¤Ć÷ŅŌ ŠĪŹ½“ęŌŚ ŠĪŹ½“ęŌŚ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com