
7��)ʵ��������һδ֪Ũ�ȵ�ϡ���ᣬijѧ����0��10 mol��L��1 NaOH����Һ���вⶨ�����Ũ�ȵ�ʵ�顣�����������գ�
ȡ20��00 mL�������������ƿ�У����μ�2��3�η�̪��ָʾ�������Լ����Ƶ�NaOH����Һ���еζ����ظ������ζ�����2��3�Σ���¼�������¡�
| ʵ�� ��� | NaOH��Һ��Ũ�� (mol��L��1) | �ζ����ʱ��NaOH��Һ��������(mL) | ������������(mL) |
| 1 | 0��10 | 22��50 | 20��00 |
| 2 | 0��10 | 25��80 | 20��00 |
| 3 | 0��10 | 22��62 | 20��00 |

��1�����һ��NaOH��Һ���룬��Һ����ɫǡ�ñ��dz��ɫ�Ұ�����ڲ���ɫ��2�֣�
��2��0��11 mol/L��2�֣� ��3�� ��(1��) ��4��D��E��2�֣�
���������������1������֪Ũ�ȵļ�ζ�δ֪Ũ�ȵ��ᣬ�÷�̪��ָʾ�������ζ��ﵽ�յ�ʱ���ῴ���μ����һ��NaOH��Һʱ����Һ����ɫ����ɫ��Ϊdz��ɫ�����������Һ����ɫ����ȥ����2����һ�����ĵ�NaOH��Һ�������22��40ml���ڶ������ĵ�NaOH��Һ�������25��70ml�����������ĵ�NaOH��Һ�������22��52ml���ɼ��ڶ���ʵ������ƫ��̫����ȥ�������ĵ�NaOH��Һ��ƽ������ǣ���22��50ml+ 22��62ml����2=22��56ml���������ǡ�÷����кͷ�Ӧʱ���ߵ����ʵ�����ȿɵã�c(HCl)= (0��10mol/L ��22��56ml) ��20��00ml=0��11 mol/L����3�� ��ʽ�ζ��ܼ��촦���ܣ�������ȥ��ʽ�ζ��������ݵķ���Ӧ������ͼ��ʾ�����еı�ͼʾ����4�� A���ζ��յ����ʱ���ӣ�ʹ����Һ���ƫС��������Ũ�Ⱦ�ƫ�ͣ�����B����ʽ�ζ���ʹ��ǰ��ˮϴ��δ�ô���������ϴ����������ʵ���ƫ�٣����ĵļ����Һ�����ƫС��ʹ�����Ũ��ƫ�ͣ�����C����ƿˮϴ��δ�����������κ�Ӱ�죬����D������NaOH�����л���Na2CO3���壬�����ĵı�־��Һ���ƫ�࣬�Ǽ��������Һ��Ũ��ƫ����ȷ��E����ʽ�ζ��ܼ��첿�������ݣ��ζ�����ʧ�������ĵļ����Һ���ƫ�����δ֪��Һ��Ũ�Ⱦ�ƫ�ߣ���ȷ��
���㣺��������к͵ζ��յ���жϡ����ݴ�����Ũ�ȵļ��㼰��������֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���л�ѧ������ȷ����
| A����ԭ�ӣ�Cl |
| B�����Ľṹ��ʽ��C6H6 |
| C��þ���ӣ�Mg2�� |
D��Neԭ�ӽṹʾ��ͼ��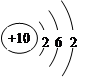 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��֪Ksp(AgCl)=1.8��10��10��Ksp(AgI)=1.5��10��16��Ksp(AgBr)=7.7��10��13�������������εı�����Һ�У�Ag+Ũ�ȴ�С˳����ȷ����
| A��AgCl>AgI> AgBr | B��AgCl> AgBr>AgI |
| C��AgBr >AgCl>AgI | D��AgBr >AgI>AgCl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��ij�¶�ʱ����ô�ˮ�е�c(H��)��2.0��10��7 mol��L��1����c(OH��)Ϊ
| A��2.0��10��7 mol��L��1 |
| B��0.1��10��7 mol��L��1 |
| C��1.0��10��14/2.0��10��7 mol��L��1 |
| D����ȷ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��10�֣���0.2000mol/L NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ������ɷֽ�Ϊ���¼�����
��A����ȡ15.00mL�����������Һע��ྻ����ƿ��������2��3�η�̪
��B���ñ�NaOH��Һ��ϴ��ʽ�ζ���2��3��
��C����ʢ�б���Һ�ĵζ��̶ܹ��ã�ʹ�ζ��ܼ��������Һ
��D��ȡ����Һע��ζ�����0�̶�����2��3cm
��E������Һ����0��0�̶����£����¶���
��F������ƿ���ڵζ������棬�ñ�NaOH��Һ�ζ����յ㣬���µζ���Һ��Ŀ̶�
������¸��գ�
��1����ȷ������˳����B��_____��_____��_____��A��F��
��2�����εζ�����NaOH��Һ���������:
| ʵ����� | 1 | 2 | 3 |
| ����NaOH��Һ�����(mL) | 20.05 | 20.00 | 19.95 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
10��)ʹ������к͵ζ����ⶨijδ֪���ʵ���Ũ�ȵ�ϡ���ᡣ
��.ʵ�鲽�裺
��1���ζ�ǰ����
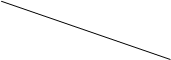
�ٵζ��ܣ�___________________��ϴ�ӡ���ϴ��װҺ�������ݡ���Һ�����¼����¼ʢװ 0.1000 mol/L NaOH��Һ�ļ�ʽ�ζ��ܵij�ʼ����ʱ��Һ��λ����ͼ��ʾ�����ʱ�Ķ���Ϊ_____________mL��
����ƿ������ʽ�ζ�����ȡ����ϡ����20.00mL����ƿ�У������еμ�2�η�̪��Һ��ָʾ����
��2���ζ�
�ñ���NaOH��Һ�ζ������ϡ����ʱ�����ֲ����ζ��ܻ�����������ҡ��ƿ���۾�ע�� ���ζ����յ�ʱ����¼NaOH��Һ���յ���������ظ��ζ�3�Ρ�
��.ʵ���¼��
 �ζ����� �ζ�����ʵ������/mL | 1 | 2 | 3 | 4 |
| V����Ʒ�� | 20.00 | 20.00 | 20.00 | 20.00 |
| V��NaOH�������ģ� | 15.80 | 15.00 | 15.02 | 14.98 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��9�֣�ijͬѧ���������ʵ��̽���ε�ˮ�ⷴӦ���ɡ�
��1����̼������Һ�е��뼸�η�̪��Һ����Һ�ʺ�ɫ�������ӷ���ʽ��ʾ��ԭ��
��2����pH��ֽ�����������Һ��pHΪ3�������ӷ���ʽ��ʾ��������Һ�����Ե�ԭ�� ��
�����ⶨ����Һ��pH�IJ��������ǣ�
��3������������Һ��̼��������Һ��ϣ��ɹ۲쵽��������������ɫ�������״������д���÷�Ӧ�����ӷ���ʽ:
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��ȡһ�����飬���õ���ѷ�����
| A�����������ȡ����Ӧ | B����ϩ�������ӳɷ�Ӧ |
| C�������HCl���� | D����ϩ��HCl�ӳɷ�Ӧ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com