����β���Ѿ���Ϊ������Ⱦ����Ҫ��ȾԴ֮һ��ij̽����ѧϰС���ͬѧ��һ�����ʵ���У���������������������ʵ�飬������ʼʱͶ���ϩ��NO���������������߳�ʱ���������壬������һϵ�б仯�������ʸ�Ӧ�����ݲɼ�����ͨ��������ó���ͼ��ʾ�ı仯���ߣ�������ߣ��Իش��������⣮
��1����������ʵ���ģ���������ֶ�����Ⱦ�����Ļ���______��
��a������������ ��b���⻯ѧ����
��c��������γ� �� ��d����ɫ��Ⱦ
��2��0��100min�ڣ�ƽ����Ӧ���������ǣ�______��ѡ�C
3H
6��NO��NO
2��ȩ��O
3��PAN��
��3����֪��
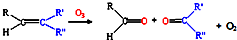
��д����ϩ�������������ȩ�Ļ�ѧ����ʽ������ƽ��______��
��4����������β����Ⱦ����NH
3ѡ���Դ���ԭ�dz��õ�һ�ַ�������950��ʱ��NH
3��NO��Ӧ���ѵ����ɵ������÷�Ӧ�Ļ�ѧ����ʽΪ��______��
��5��2000Kʱ�����Է������·�Ӧ��
1/2N
2+1/2O
2?NO��K
11/2N
2+O
2?NO
2��K
24NO?2NO
2+N
2���÷�Ӧ��ƽ�ⳣ��K
3=______����K
1��K
2��ʾ����


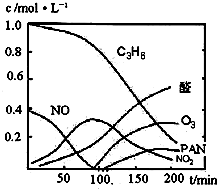



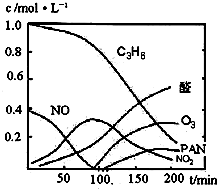 ����β���Ѿ���Ϊ������Ⱦ����Ҫ��ȾԴ֮һ��ij̽����ѧϰС���ͬѧ��һ�����ʵ���У���������������������ʵ�飬������ʼʱͶ���ϩ��NO���������������߳�ʱ���������壬������һϵ�б仯�������ʸ�Ӧ�����ݲɼ�����ͨ��������ó���ͼ��ʾ�ı仯���ߣ�������ߣ��Իش��������⣮
����β���Ѿ���Ϊ������Ⱦ����Ҫ��ȾԴ֮һ��ij̽����ѧϰС���ͬѧ��һ�����ʵ���У���������������������ʵ�飬������ʼʱͶ���ϩ��NO���������������߳�ʱ���������壬������һϵ�б仯�������ʸ�Ӧ�����ݲɼ�����ͨ��������ó���ͼ��ʾ�ı仯���ߣ�������ߣ��Իش��������⣮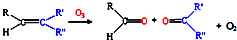 ��д����ϩ�������������ȩ�Ļ�ѧ����ʽ������ƽ��
��д����ϩ�������������ȩ�Ļ�ѧ����ʽ������ƽ�� 9N2��g��+6CO2��g��+6H2O��g����
9N2��g��+6CO2��g��+6H2O��g���� 9N2��g��+12CO2��g��+12H2O��g����
9N2��g��+12CO2��g��+12H2O��g����
 O2+Hb?CO K=220
O2+Hb?CO K=220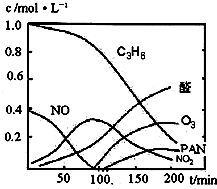 ����β���Ѿ���Ϊ������Ⱦ����Ҫ��ȾԴ֮һ��ij̽����ѧϰС���ͬѧ��һ�����ʵ���У���������������������ʵ�飬������ʼʱͶ���ϩ��NO���������������߳�ʱ���������壬������һϵ�б仯�������ʸ�Ӧ�����ݲɼ�����ͨ��������ó���ͼ��ʾ�ı仯���ߣ�������ߣ��Իش��������⣮
����β���Ѿ���Ϊ������Ⱦ����Ҫ��ȾԴ֮һ��ij̽����ѧϰС���ͬѧ��һ�����ʵ���У���������������������ʵ�飬������ʼʱͶ���ϩ��NO���������������߳�ʱ���������壬������һϵ�б仯�������ʸ�Ӧ�����ݲɼ�����ͨ��������ó���ͼ��ʾ�ı仯���ߣ�������ߣ��Իش��������⣮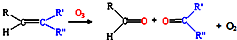 ��д����ϩ�������������ȩ�Ļ�ѧ����ʽ������ƽ��______��
��д����ϩ�������������ȩ�Ļ�ѧ����ʽ������ƽ��______�� ��д����ϩ�������������ȩ�Ļ�ѧ����ʽ������ƽ��______��
��д����ϩ�������������ȩ�Ļ�ѧ����ʽ������ƽ��______��