
·ÖĪö ¢ŁČÜŅŗ³ŹĒæĖįŠŌ£¬ĖµĆ÷ČÜŅŗÖŠ“ęŌŚH+£»ŅĄ¾ŻĄė×Ó¹²“ęæÉÖŖŅ»¶Ø²»“ęŌŚCO32-£¬AlO2-£»
¢ŚČ”ČÜŅŗŹŹĮ棬¼ÓČėÉŁĮæCCl4ŗĶŹżµĪŠĀÖĘĀČĖ®£¬Õńµ“£¬CCl4²ć³Ź×ĻŗģÉ«£¬ĖµĆ÷ČÜŅŗÖŠŅ»¶Øŗ¬ÓŠI-£¬ŅĄ¾ŻĄė×Ó¹²“ę·ÖĪöÅŠ¶ĻæÉÖŖ£¬Ņ»¶Ø²»ŗ¬ÓŠFe3+”¢NO3-£»
¢ŪĮķČ”ČÜŅŗŹŹĮ棬֚µĪ¼ÓČėNaOHČÜŅŗ£»
a£®ČÜŅŗ“ÓĖįŠŌ±äĪŖÖŠŠŌ£¬ÖŠŗĶĖį£»
b£®ČÜŅŗÖš½„²śÉś³Įµķ£¬·ÖĪöŃōĄė×ÓÖŠÖ»ÓŠĀĮĄė×Ó³Įµķ£»
c£®³ĮµķĶźČ«Čܽā£¬Ö¤Ć÷Éś³ÉµÄ³ĮµķŹĒĒāŃõ»ÆĀĮ£¬ŌČÜŅŗÖŠŅ»¶Øŗ¬ÓŠAl3+£»
d£®×īŗó¼ÓČČČÜŅŗ£¬ÓŠĘųĢå·Å³ö£¬øĆĘųĢåÄÜŹ¹ŹŖČóµÄŗģÉ«ŹÆČļŹŌÖ½±äĄ¶£¬Ö¤Ć÷Éś³ÉµÄĘųĢåŹĒ°±Ęų£¬ŌČÜŅŗÖŠŅ»¶Øŗ¬ÓŠļ§øłĄė×Ó£»
¢ÜČ”ŹŹĮæ¢ŪµĆµ½µÄ¼īŠŌČÜŅŗ£¬ĀĮĄė×Ó·“Ӧɜ³ÉĘ«ĀĮĖįøłĄė×Ó£¬¼ÓČėNa2CO3ČÜŅŗ£¬ÓŠ°×É«³ĮµķÉś³É£¬·ÖĪöĄė×ÓæÉÖŖÖ»ÓŠ±µĄė×ÓÉś³ÉĢ¼Ėį±µ³Įµķ£¬ĖµĆ÷ŌČÜŅŗÖŠ²»ŗ¬ÓŠĮņĖįøłĄė×Ó£»
·ÖĪöĶʶĻĄė×ӵēęŌŚĒéæö»Ų“šĪŹĢā£®
½ā“š ½ā£ŗ¢ŁÓĆpHŹŌÖ½¼ģŃ飬ČÜŅŗ³ŹĒæĖįŠŌ£¬ČÜŅŗÖŠ“ęŌŚ“óĮæH+£¬ÓÉÓŚĢ¼ĖįøłĄė×ÓŗĶĘ«ĀĮĖįøłĄė×Ó¶¼ŹĒČõĖįŅõĄė×ÓŗĶĒāĄė×Ó·“Ó¦£¬ĖłŅŌCO32-£¬AlO2- ²»ÄÜ“óĮæ“ęŌŚ£»
¢ŚČ”ČÜŅŗŹŹĮ棬¼ÓČėÉŁĮæCCl4ŗĶŹżµĪŠĀÖʵÄĀČĖ®Õńµ“£¬ĖÄĀČ»ÆĢ¼²ć³Ź×ĻÉ«£¬ĖµĆ÷ČÜŅŗÖŠŗ¬ÓŠI-£¬ÓÉÓŚ Fe3+”¢NO3-£ØŌŚĖįČÜŅŗÖŠ£©ÄÜŃõ»ÆI-ĪŖI2£¬ĖłŅŌČÜŅŗÖŠ²»“ęŌŚFe3+”¢NO3-£»
¢ŪŅĄ¾ŻĢāŅāÉś³ÉµÄ³ĮµķÓÖČܽā£¬ĖµĆ÷ČÜŅŗÖŠ“ęŌŚAl3+£¬ŅĄ¾Ż¼ÓČČČÜŅŗÉś³ÉµÄĘųĢåÄÜŹ¹ŹŖČóµÄŗģÉ«ŹÆČļŹŌÖ½±äĄ¶Ö¤Ć÷ĘųĢåŹĒ°±Ęų£¬ĖµĆ÷ŌČÜŅŗÖŠŗ¬ÓŠNH4+£»
¢ÜČ”ŹŹĮæ¢ŪµĆµ½µÄ¼īŠŌČÜŅŗ£¬¼ÓČėĢ¼ĖįÄĘČÜŅŗÓŠ°×É«³ĮµķÉś³É£¬ĖµĆ÷ČÜŅŗÖŠ“ęŌŚ±µĄė×Ó£¬ŌņŅ»¶Ø²»“ęŌŚĮņĖįøłĄė×Ó£»
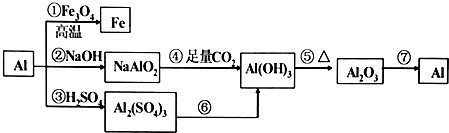
£Ø1£©ÓÉ¢ŁæÉŅŌÅųżCO32-£¬AlO2-µÄ“ęŌŚ£¬¹Ź“šĪŖ£ŗCO32-£¬AlO2-£»
£Ø2£©ÓÉ¢ŚæÉŅŌÖ¤Ć÷I-Ņ»ŃłµÄ“ęŌŚ£¬CCl4²ć³öĻÖµāµ„ÖŹµÄŃÕÉ«Ö¤Ć÷ŗ¬I-£¬Fe3+”¢NO3-ŌŚøĆ»·¾³ÖŠÓėI-²»Äܹ²“ę£¬ŅĄ¾ŻĄė×Ó¹²“ęĶ¬Ź±ÅųżFe3+”¢NO3-Ąė×ӵēęŌŚ£¬
¹Ź“š°øĪŖ£ŗI-£»Fe3+”¢NO3-£»
£Ø3£©¢ŪÖ¤Ć÷ČÜŅŗÖŠŅ»¶Øŗ¬ÓŠAl3+£¬NH4+£»c”¢dĖłÉę¼°µÄ»Æѧ·“Ó¦ĪŖĒāŃõ»ÆĀĮČܽāÓŚĒāŃõ»ÆÄĘČÜŅŗÖŠÉś³ÉĘ«ĀĮĖįÄĘ£¬Ņ»Ė®ŗĻ°±ŹÜČČ·Ö½āÉś³É°±Ęų£¬ŹĒĄė×Ó·“Ó¦µÄÓĆĄė×Ó·½³ĢŹ½±ķŹ¾ĪŖ£ŗAl£ØOH£©3+OH-=AlO2-+H2O£¬NH3•H2O$\frac{\underline{\;¼ÓČČ\;}}{\;}$NH3”ü+H2O£¬
¹Ź“š°øĪŖ£ŗAl3+”¢NH4+£»Al£ØOH£©3+OH-=AlO2-+H2O£»NH3•H2O$\frac{\underline{\;¼ÓČČ\;}}{\;}$NH3”ü+H2O£»
£Ø4£©¢ÜŹµŃé·ÖĪöÅŠ¶Ļ£¬Č”ŹŹĮæ¢ŪµĆµ½µÄ¼īŠŌČÜŅŗ£¬ĀĮĄė×Ó·“Ӧɜ³ÉĘ«ĀĮĖįøłĄė×Ó£¬¼ÓČėNa2CO3ČÜŅŗ£¬ÓŠ°×É«³ĮµķÉś³É£¬·ÖĪöĄė×ÓæÉÖŖÖ»ÓŠ±µĄė×ÓÉś³ÉĢ¼Ėį±µ³Įµķ£¬ĖµĆ÷ŌČÜŅŗÖŠ²»ŗ¬ÓŠĮņĖįøłĄė×Ó£»
¹Ź“š°øĪŖ£ŗSO42-£®
µćĘĄ ±¾Ģāæ¼²éĮĖĄė×Ó¼ģŃéµÄŹµŃé·½·ØŗĶ·“Ó¦ĻÖĻóµÄÅŠ¶Ļ£¬Ąė×ÓŠŌÖŹŗĶĄė×Ó·“Ó¦½ųŠŠµÄĄė×Ó¹²“ę·ÖĪöŹĒ½āĢā¹Ų¼ü£¬ĢāÄæÄѶČÖŠµČ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 3£¬3-¶ž¼×»ł¶”Ķé | B£® | 2£¬3-¶ž¼×»ł-4-ŅŅ»ł¼ŗĶé | ||
| C£® | 2£¬3£¬3-Čż¼×»ł¶”Ķé | D£® | 2£¬2-¶ž¼×»ł-3-ŅŅ»ł¶”Ķé |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 2£¬3-¶ž¼×»ł¶”Ķé | B£® | 3£¬3-¶ž¼×»łĪģĶé | ||
| C£® | 3-¼×»ł-2-ŅŅ»łĪģĶé | D£® | 2£¬2£¬3£¬3-Ėļ׻ł¶”Ķé |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ÄŃŹ§µē×ÓµÄŌ×Ó£¬»ńµĆµē×ÓµÄÄÜĮ¦Ņ»¶ØĒæ | |
| B£® | Ņ׵Ƶē×ÓµÄŌ×ÓĖłŠĪ³ÉµÄ¼ņµ„ŅõĄė×Ó£¬Ę仹ŌŠŌŅ»¶ØĒæ | |
| C£® | »īĘĆ½šŹōÓė»īĘĆ·Ē½šŹō»ÆŗĻ£¬Ņ׊Ī³ÉĄė×Ó¼ü | |
| D£® | µē×Ó²ć½į¹¹ĻąĶ¬µÄ²»Ķ¬Ąė×Ó£¬ŗĖµēŗÉŹżŌ½¶ą£¬°ė¾¶Ō½“ó |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
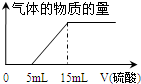 ij»ÆѧŠĖȤŠ”×éÓĆ100mL 1mol/L NaOHČÜŅŗĶźČ«ĪüŹÕĮĖa mol CO2ŗóµĆµ½ČÜŅŗA£ØŅŗĢåĢå»żĪŽ±ä»Æ£©£®ĪŖĮĖČ·¶ØČÜŅŗAµÄČÜÖŹ³É·Ö¼°aÖµ£¬øĆŠĖȤŠ”×éµÄĶ¬Ń§½ųŠŠĮĖČēĻĀŹµŃ飮Ēė°ļÖśĖūĆĒĶź³ÉĻĀĮŠĻąÓ¦ŹµŃéÄŚČŻ£®
ij»ÆѧŠĖȤŠ”×éÓĆ100mL 1mol/L NaOHČÜŅŗĶźČ«ĪüŹÕĮĖa mol CO2ŗóµĆµ½ČÜŅŗA£ØŅŗĢåĢå»żĪŽ±ä»Æ£©£®ĪŖĮĖČ·¶ØČÜŅŗAµÄČÜÖŹ³É·Ö¼°aÖµ£¬øĆŠĖȤŠ”×éµÄĶ¬Ń§½ųŠŠĮĖČēĻĀŹµŃ飮Ēė°ļÖśĖūĆĒĶź³ÉĻĀĮŠĻąÓ¦ŹµŃéÄŚČŻ£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ±ź×¼×“æöĻĀ£¬22.4LH2Oŗ¬ÓŠµÄ·Ö×ÓŹżĪŖNA | |
| B£® | 28gN2ŗĶN4×é³ÉµÄ»ģŗĻĘųĢåÖŠŗ¬ÓŠµÄŌ×ÓŹżĪŖ2NA | |
| C£® | 1mol•L-1AlCl3ČÜŅŗÖŠŗ¬ÓŠµÄCl-ŹżĪŖ3NA | |
| D£® | ½«1mol CO2ČÜÓŚ1LĖ®ÖŠ£¬ĖłµĆČÜŅŗÖŠĖłŗ¬·Ö×ÓŹżĪŖNA |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com