
| ×å ÖÜĘŚ | ¢ńA | ¢ņA | ¢óA | ¢ōA | ¢õA | ¢öA | ¢ö¢ńA |
| 2 | ¢Ł | ¢Ś | ¢Ū | ||||
| 3 | ¢Ü | ¢Ż | ¢Ž | ¢ß | ¢ą |
·ÖĪö ÓÉŌŖĖŲŌŚÖÜĘŚ±ķµÄĪ»ÖĆæÉÖŖ£¬¢ŁŹĒC£¬¢ŚŹĒN£¬¢ŪŹĒO£¬¢ÜŹĒNa£¬¢ŻŹĒAl£¬¢ŽŹĒSi£¬¢ßŹĒS£¬¢ąŹĒCl£¬
£Ø1£©CŠĪ³ÉÓŠ»śĪļ£¬ÖÖĄą×ī¶ą£»
£Ø2£©Ģ¼ĖįµÄĖįŠŌ±Č¹čĖįµÄĖįŠŌĒ棬æÉĖµĆ÷C±ČSiµÄ·Ē½šŹōŠŌĒ棻
£Ø3£©°±ĘųÓėĀČĘų·“Ӧɜ³ÉµŖĘųŗĶHCl£»
£Ø4£©ŌŖĖŲ¢ŪŗĶ¢ÜŠĪ³ÉµÄ»ÆŗĻĪļÖŠæÉŅŌ×ö¹©Ńõ¼Į”¢ĘÆ°×¼ĮµÄŹĒ¹żŃõ»ÆÄĘ£»
£Ø5£©ŌŖĖŲ¢Ū”¢¢ŻŗĶ¢ßŠĪ³ÉµÄ»ÆŗĻĪļĪŖĮņĖįĀĮ£¬³£ÓĆÓŚ¹¤ŅµĪŪĖ®µÄ“¦Ąķ£¬ÓėĀĮĄė×ÓĖ®½āÉś³É½ŗĢåÓŠ¹Ų£®
½ā“š ½ā£ŗÓÉŌŖĖŲŌŚÖÜĘŚ±ķµÄĪ»ÖĆæÉÖŖ£¬¢ŁŹĒC£¬¢ŚŹĒN£¬¢ŪŹĒO£¬¢ÜŹĒNa£¬¢ŻŹĒAl£¬¢ŽŹĒSi£¬¢ßŹĒS£¬¢ąŹĒCl£¬
£Ø1£©ÉĻŹöŌŖĖŲÖŠ£¬ŠĪ³É»ÆŗĻĪļÖÖĄą×ī¶ąµÄŹĒC£¬¹Ź“š°øĪŖ£ŗC£»
£Ø2£©ŌŖĖŲ¢ŁµÄ·Ē½šŹōŠŌ±ČŌŖĖŲ¢ŽĒ棬æÉŅŌÓĆĄ“Ö¤Ć÷øĆ½įĀŪµÄŹĀŹµŹĒĢ¼ĖįµÄĖįŠŌ±Č¹čĖįµÄĖįŠŌĒ棬¹Ź“š°øĪŖ£ŗĢ¼ĖįµÄĖįŠŌ±Č¹čĖįµÄĖįŠŌĒ棻
£Ø3£©°±ĘųÓėĀČĘų·“Ӧɜ³ÉµŖĘųŗĶHCl£¬·“Ó¦ĪŖ2NH3+Cl2=N2+6HCl£¬¹Ź“š°øĪŖ£ŗ2NH3+Cl2=N2+6HCl£»
£Ø4£©ŌŖĖŲ¢ŪŗĶ¢ÜŠĪ³ÉµÄ»ÆŗĻĪļÖŠæÉŅŌ×ö¹©Ńõ¼Į”¢ĘÆ°×¼ĮµÄŹĒ¹żŃõ»ÆÄĘ£¬Ęä»ÆѧŹ½ĪŖNa2O2£¬¹Ź“š°øĪŖ£ŗNa2O2£»
£Ø5£©ŌŖĖŲ¢Ū”¢¢ŻŗĶ¢ßŠĪ³ÉµÄ»ÆŗĻĪļĪŖĮņĖįĀĮ£¬³£ÓĆÓŚ¹¤ŅµĪŪĖ®µÄ“¦Ąķ£¬ĘäÖ÷ŅŖ×÷ÓĆĪŖĀĮĄė×ÓĖ®½āÉś³É½ŗĢå¾ßÓŠĪüø½ŠŌ£¬æɾ»»ÆĖ®£¬
¹Ź“š°øĪŖ£ŗĀĮĄė×ÓĖ®½āÉś³É½ŗĢå¾ßÓŠĪüø½ŠŌ£¬æɾ»»ÆĖ®£®
µćĘĄ ±¾Ģāæ¼²éĪ»ÖĆ”¢½į¹¹ÓėŠŌÖŹ£¬ĪŖøßĘµæ¼µć£¬°ŃĪÕŌŖĖŲµÄĪ»ÖĆ”¢ŌŖĖŲµÄŠŌÖŹ”¢ŌŖĖŲ»ÆŗĻĪļÖŖŹ¶ĪŖ½ā“šµÄ¹Ų¼ü£¬²ąÖŲ·ÖĪöÓėÓ¦ÓĆÄÜĮ¦µÄ漲飬עŅāŌŖĖŲ»ÆŗĻĪļ¼°Ļą¹Ų·“Ó¦ŌĄķµÄÓ¦ÓĆ£¬ĢāÄæÄŃ¶Č²»“ó£®


 ĆūŹ¦µć²¦¾ķĻµĮŠ“š°ø
ĆūŹ¦µć²¦¾ķĻµĮŠ“š°ø Ó¢²Å¼Ę»®ĘŚÄ©µ÷ŃŠĻµĮŠ“š°ø
Ó¢²Å¼Ę»®ĘŚÄ©µ÷ŃŠĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 ČēĶ¼ŹĒijĶ¬Ń§Éč¼ĘµÄŅ»øö¼ņŅ×µÄŌµē³Ų×°ÖĆ£¬»Ų“šĻĀĮŠĪŹĢā£®
ČēĶ¼ŹĒijĶ¬Ń§Éč¼ĘµÄŅ»øö¼ņŅ×µÄŌµē³Ų×°ÖĆ£¬»Ų“šĻĀĮŠĪŹĢā£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĪŖ¼ģŃéĀ±“śĢžÖŠµÄĀ±Ō×Ó£¬ĻČ¼ÓČėNaOHČÜŅŗ¼ÓČČ£¬ŌŁ¼ÓČėAgNO3ČÜŅŗ¹Ū²ģŃÕÉ«±ä»Æ | |
| B£® | ĪŖÖĘČ”ŅŅĖįŅŅõ„£¬½«Ļ”H2SO4ŗĶŅŅ“¼”¢ŅŅĖį»ģŗĻ¼ÓČČ£¬·Å³öµÄÕōĘųĶØČėŹ¢ÓŠNaOHČÜŅŗµÄŹŌ¹ÜÖŠ | |
| C£® | ĪŖ¼ģŃéČ©»łµÄ“ęŌŚ£¬ĻČĻņŹŌ¹ÜÖŠ¼ÓČė2mL2%µÄNaOHČÜŅŗŗĶŹżµĪCuSO4ČÜŅŗ£¬ŌŁ¼ÓŅŅČ©£¬Öó·Š | |
| D£® | ĪŖÖĘČ”ŅŅĻ©£¬½«Ļ”H2SO4ŗĶŅŅ“¼»ģŗĻ¼ÓČČ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¼ĘĖćĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | øı䷓ӦĪļÓĆĮæ | B£® | Ōö“óŃ¹Ēæ | C£® | Ōö“óĢå»ż | D£® | ÉżøßĪĀ¶Č |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | BCl3 | B£® | NCl3 | C£® | H2S | D£® | BeCl2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| ŃōĄė×Ó | K+”¢Na+”¢NH4+”¢Fe2+”¢Ba2+”¢Cu2+ |
| ŅõĄė×Ó | OH-”¢I-”¢NO3-”¢AlO2-”¢HCO3-”¢HSO4- |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A | B | C | D | |
| ·½°ø | 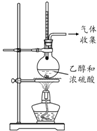 |  | 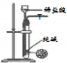 | 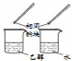 |
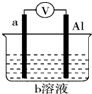
| ÄæµÄ | ĄūÓĆŅŅ“¼µÄĻūČ„·“Ó¦ÖĘČ”ŅŅĻ© | Õō·¢NH4Cl±„ŗĶČÜŅŗÖʱøNH4Cl¾§Ģå | ÖĘȔɣĮæ“æ¾»µÄCO2ĘųĢå | ±Č½ĻŅŅ“¼ÖŠōĒ»łĒāŌ×ÓŗĶĖ®·Ö×ÓÖŠĒāŌ×ӵĻīĘĆŠŌ |
| A£® | A | B£® | B | C£® | C | D£® | D |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com