
Cu�Ļ��������������������Ҫ���ã���ͬ��Ӧ���Ƶò�ͬ״̬��Cu2O
��1����ѧ�о�����������Cu2O����Ϊ̫����ֽ�ˮ�Ĵ�����
���ڼ�����������Һ̬�£�N2H4����ԭ����Cu��OH��2���Ʊ�����Cu2O��ͬʱ�ų�N2�����ռ���N2���Ϊ3.36L���ѻ���Ϊ��״����ʱ�����Ʊ�����Cu2O������Ϊ ��
��һ���¶��£���2 L�ܱ������м�������Cu2O��ͨ��0.20 molˮ������������Ӧ��
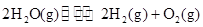
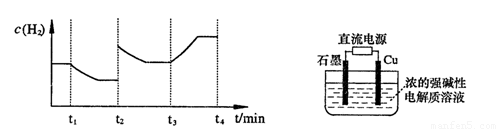
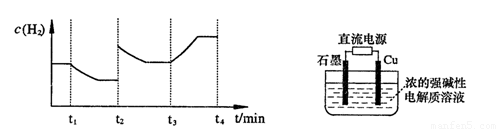
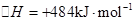 �����20 minʱO2�����ʵ���Ϊ0.0016 mol����ǰ20 min�ķ�Ӧ����v��H2O��= �����¶��£���Ӧ��ƽ�ⳣ������ʽK= ����ͼ��ʾ��t1ʱ�̴ﵽƽ���ֻ�ı�һ�������ִﵽƽ��IJ�ͬʱ���ڣ�H2��Ũ����ʱ��仯���������t1ʱƽ����ƶ�����Ϊ
��t2ʱ�ı����������Ϊ
������K1��K2��K3�ֱ��ʾt1ʱ����ı�����������ʱ����ڵ�ƽ�ⳣ����t3ʱ��û�м���������ϵ�е��κ����ʣ���K1��K2��K3�Ĺ�ϵΪ
��
�����20 minʱO2�����ʵ���Ϊ0.0016 mol����ǰ20 min�ķ�Ӧ����v��H2O��= �����¶��£���Ӧ��ƽ�ⳣ������ʽK= ����ͼ��ʾ��t1ʱ�̴ﵽƽ���ֻ�ı�һ�������ִﵽƽ��IJ�ͬʱ���ڣ�H2��Ũ����ʱ��仯���������t1ʱƽ����ƶ�����Ϊ
��t2ʱ�ı����������Ϊ
������K1��K2��K3�ֱ��ʾt1ʱ����ı�����������ʱ����ڵ�ƽ�ⳣ����t3ʱ��û�м���������ϵ�е��κ����ʣ���K1��K2��K3�Ĺ�ϵΪ
��
��2����֪�� ��H����293kJ��mol��1
��H����293kJ��mol��1
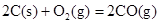 ��H����221kJ��mol��1
��H����221kJ��mol��1
��д��������̿�ۻ�ԭCuO��s���Ʊ�Cu2O��s�����Ȼ�ѧ����ʽ ��
��3���õ�ⷨҲ���Ʊ�Cu2O��ԭ��������ͼ��ʾ���������缫��Ӧ���Ա�ʾΪ ��
 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
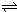 2H2��g��+O2��g����H=+484kJ?mol-1�����20minʱO2�����ʵ���Ϊ0.0016mol����ǰ20min�ķ�Ӧ����v��H2O��=
2H2��g��+O2��g����H=+484kJ?mol-1�����20minʱO2�����ʵ���Ϊ0.0016mol����ǰ20min�ķ�Ӧ����v��H2O��=| c2(H2)?c(O2) |
| c2(H2O) |
| c2(H2)?c(O2) |
| c2(H2O) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Cu�Ļ��������������������Ҫ���ã���ͬ��Ӧ���Ƶò�ͬ״̬��Cu2O
��1����ѧ�о�����������Cu2O����Ϊ̫����ֽ�ˮ�Ĵ�����
���ڼ�����������Һ̬�£�N2H4����ԭ����Cu��OH��2���Ʊ�����Cu2O��ͬʱ�ų�N2�����ռ���N2���Ϊ3.36L���ѻ���Ϊ��״����ʱ�����Ʊ�����Cu2O������Ϊ ��
��һ���¶��£���2 L�ܱ������м�������Cu2O��ͨ��0.20 molˮ������������Ӧ��
�����20 minʱO2�����ʵ���Ϊ0.0016 mol����ǰ20 min�ķ�Ӧ����v��H2O��= �����¶��£���Ӧ��ƽ�ⳣ������ʽK= ����ͼ��ʾ��t1ʱ�̴ﵽƽ���ֻ�ı�һ�������ִﵽƽ��IJ�ͬʱ���ڣ�H2��Ũ����ʱ��仯���������t1ʱƽ����ƶ�����Ϊ ��t2ʱ�ı����������Ϊ ������K1��K2��K3�ֱ��ʾt1ʱ����ı�����������ʱ����ڵ�ƽ�ⳣ����t3ʱ��û�м���������ϵ�е��κ����ʣ���K1��K2��K3�Ĺ�ϵΪ ��
��2����֪����H����293kJ��mol��1
��H����221kJ��mol��1
��д��������̿�ۻ�ԭCuO��s���Ʊ�Cu2O��s�����Ȼ�ѧ����ʽ ��
��3���õ�ⷨҲ���Ʊ�Cu2O��ԭ��������ͼ��ʾ���������缫��Ӧ���Ա�ʾΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�콭��ʡ������ѧ������Ӧ�Կ��ԣ����ƣ���ѧ���� ���ͣ������
Cu�Ļ��������������������Ҫ���ã���ͬ��Ӧ���Ƶò�ͬ״̬��Cu2O
��1����ѧ�о�����������Cu2O����Ϊ̫����ֽ�ˮ�Ĵ�����
���ڼ�����������Һ̬�£�N2H4����ԭ����Cu��OH��2���Ʊ�����Cu2O��ͬʱ�ų�N2�����ռ���N2���Ϊ3.36L���ѻ���Ϊ��״����ʱ�����Ʊ�����Cu2O������Ϊ ��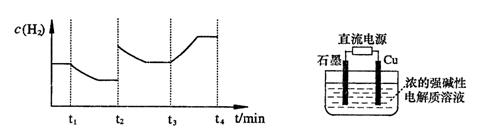
��һ�� �¶��£���2 L�ܱ������м�������Cu2O��ͨ��0.20 molˮ������������Ӧ��
�¶��£���2 L�ܱ������м�������Cu2O��ͨ��0.20 molˮ������������Ӧ��
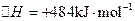 �����20 minʱO2�����ʵ���Ϊ0.0016 mol����ǰ20 min�ķ�Ӧ����v��H2O��= �����¶��£���Ӧ��ƽ�ⳣ������ʽK= ����ͼ��ʾ��t1ʱ�̴ﵽƽ���ֻ�ı�һ�������ִﵽƽ��IJ�ͬʱ���ڣ�H2��Ũ����ʱ��仯���������t1ʱƽ����ƶ�����Ϊ ��t2ʱ�ı����������Ϊ ������K1��K2��K3�ֱ��ʾt1ʱ����ı�����������ʱ����ڵ�ƽ
�����20 minʱO2�����ʵ���Ϊ0.0016 mol����ǰ20 min�ķ�Ӧ����v��H2O��= �����¶��£���Ӧ��ƽ�ⳣ������ʽK= ����ͼ��ʾ��t1ʱ�̴ﵽƽ���ֻ�ı�һ�������ִﵽƽ��IJ�ͬʱ���ڣ�H2��Ũ����ʱ��仯���������t1ʱƽ����ƶ�����Ϊ ��t2ʱ�ı����������Ϊ ������K1��K2��K3�ֱ��ʾt1ʱ����ı�����������ʱ����ڵ�ƽ �ⳣ����t3ʱ��û�м���������ϵ�е��κ����ʣ���K1��K2��K3�Ĺ�ϵΪ ��
�ⳣ����t3ʱ��û�м���������ϵ�е��κ����ʣ���K1��K2��K3�Ĺ�ϵΪ ��
��2����֪��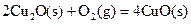 ��H����293kJ��mol��1
��H����293kJ��mol��1 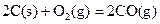 ��H����221kJ��mol��1
��H����221kJ��mol��1
��д��������̿�ۻ�ԭCuO��s���Ʊ�Cu2O��s�����Ȼ�ѧ����ʽ ��
��3���õ�ⷨҲ���Ʊ�Cu2O��ԭ��������ͼ��ʾ���������缫��Ӧ���Ա�ʾΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�꽭��ʡ������Ӧ�Կ��ԣ����ƣ���ѧ���� ���ͣ������
Cu�Ļ��������������������Ҫ���ã���ͬ��Ӧ���Ƶò�ͬ״̬��Cu2O
��1����ѧ�о�����������Cu2O����Ϊ̫����ֽ�ˮ�Ĵ�����
���ڼ�����������Һ̬�£�N2H4����ԭ����Cu��OH��2���Ʊ�����Cu2O��ͬʱ�ų�N2�����ռ���N2���Ϊ3.36L���ѻ���Ϊ��״����ʱ�����Ʊ�����Cu2O������Ϊ ��
��һ���¶��£���2 L�ܱ������м�������Cu2O��ͨ��0.20 molˮ������������Ӧ��
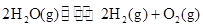
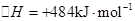 �����20 minʱO2�����ʵ���Ϊ0.0016 mol����ǰ20 min�ķ�Ӧ����v��H2O��= �����¶��£���Ӧ��ƽ�ⳣ������ʽK= ����ͼ��ʾ��t1ʱ�̴ﵽƽ���ֻ�ı�һ�������ִﵽƽ��IJ�ͬʱ���ڣ�H2��Ũ����ʱ��仯���������t1ʱƽ����ƶ�����Ϊ
��t2ʱ�ı����������Ϊ
������K1��K2��K3�ֱ��ʾt1ʱ����ı�����������ʱ����ڵ�ƽ�ⳣ����t3ʱ��û�м���������ϵ�е��κ����ʣ���K1��K2��K3�Ĺ�ϵΪ
��
�����20 minʱO2�����ʵ���Ϊ0.0016 mol����ǰ20 min�ķ�Ӧ����v��H2O��= �����¶��£���Ӧ��ƽ�ⳣ������ʽK= ����ͼ��ʾ��t1ʱ�̴ﵽƽ���ֻ�ı�һ�������ִﵽƽ��IJ�ͬʱ���ڣ�H2��Ũ����ʱ��仯���������t1ʱƽ����ƶ�����Ϊ
��t2ʱ�ı����������Ϊ
������K1��K2��K3�ֱ��ʾt1ʱ����ı�����������ʱ����ڵ�ƽ�ⳣ����t3ʱ��û�м���������ϵ�е��κ����ʣ���K1��K2��K3�Ĺ�ϵΪ
��
��2����֪��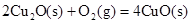 ��H����293kJ��mol��1
��H����293kJ��mol��1
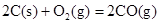 ��H����221kJ��mol��1
��H����221kJ��mol��1
��д��������̿�ۻ�ԭCuO��s���Ʊ�Cu2O��s�����Ȼ�ѧ����ʽ ��
��3���õ�ⷨҲ���Ʊ�Cu2O��ԭ��������ͼ��ʾ���������缫��Ӧ���Ա�ʾΪ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com