
��16�֣�ʳ�����ճ�����ı���Ʒ��Ҳ����Ҫ�Ļ���ԭ�ϡ�
(1)��ʳ�γ�������K����Ca2����Mg2����Fe3����SO42�����������ӣ�ʵ�����ᴿNaCl���������£�
�ṩ���Լ�������Na2CO3��Һ������K2CO3��Һ��NaOH��Һ��BaCl2��Һ��Ba(NO3)2��Һ �� 75%�Ҵ������Ȼ�̼������ȥ��ҺI�е�Ca2����Mg2����Fe3����SO42�����ӣ�ѡ��a���������Լ������μ�˳�������� ��ֻ�ѧʽ����
(2)���ᴿ��NaCl����500 mL 4.00 mol��L-1 NaCl��Һ������������ҩ�ס���������������ƽ���ձ�����Ҫ �����������ƣ���������ʱ���ӿ̶��ߣ���������Һ��Ũ�� ���ƫ����ƫС������Ӱ�족����
(3)�ڵ�ⱥ��ʳ��ˮ��ʵ���У����ռ���H2Ϊ3.36 L���ڱ�״���£�����ת�Ƶ��ӵĸ���Ϊ ��ͬ���������ռ���Cl2 �����������=��������3.36 L��
(4)ʵ�����Ʊ�Cl2ͨ�����ö���������Ũ���Ṳ������ȡ��������Ӧ�����ӷ���ʽΪ�� ��
�ݴˣ���������������װ����ѡ���Ʊ����ռ��������Cl2��װ�� ������ţ�
��ѡ���Ʊ������װ�ã�
��ѡװ���е�����ϴ��ƿ��Ӧ����ʢװ �� �����Լ������ƣ������ϻ�ѧƽ���ƶ�ԭ�����͵�һ��ϴ��ƿ���Լ�ѡ������� ��β���� ��Һ���ѧʽ�����գ�д��������Ӧ�����ӷ���ʽ ��
��16�֣�
��1�� BaCl2��NaOH ��Na2CO3��2�֣���������˳��Ҳ���֣�
��2��500 mL������ƿ����ͷ�ιܣ�2�֣�д�벻д����Ͳ�������۷֣�
ƫС��1�֣�
��3��0.3NA��1.806��1023��1�� ��
< ��1�֣�
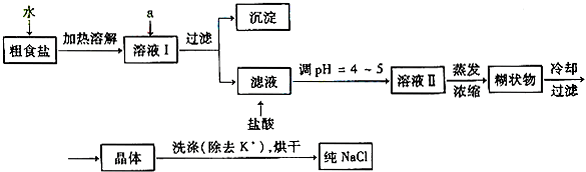
��4��MnO2��4H����2Cl��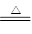 Mn2����Cl2����2H2O��2�� ��
Mn2����Cl2����2H2O��2�� ��
d ��1�� ��
����ʳ��ˮ�͵��Ȼ�����Һ��Ũ���ᣨ2�� ��
Cl2����ˮ����ˮ������ӦCl2 +H2O  H++Cl��+HClO,����Cl����Ũ��ʹƽ�������ƶ����������ռ���������� ��2�� �� NaOH ��1�� �� Cl����2OH����Cl����ClO����H2O��2�� ��
H++Cl��+HClO,����Cl����Ũ��ʹƽ�������ƶ����������ռ���������� ��2�� �� NaOH ��1�� �� Cl����2OH����Cl����ClO����H2O��2�� ��
����


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1��ը����ʱ1 kg��������0.5 kgˮ��4 g������10 gС�մ�����ʳ�εȸ��ϣ�����ը����Ʒ�����IJ���һ��Ϊ80%��ͨ������˵����ÿ��ʳ��100 g��������������������__________________��?
��2�����о��ҹ��������ճ�����������������ʳƷ���Ӽ��⣩�����ֿ���;����______________________________________________________________________?
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��0115 �¿��� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com