
| m |
| V |
| 1 |
| 8 |
| 1 |
| 6 |
| ||
| (a��10-10)3 |
| ||
| (a��10-10)3 |


 ����С��ʿ���������ϵ�д�
����С��ʿ���������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ȡһС������ƣ�����ȼ�ճ�����ȣ����������ۻ���ȼ��ʱ����Ϊ��ɫ�����ɵ���ɫ�������� |
| B����ȥNaHCO3��Һ�е�Na2CO3��Ӧͨ�������CO2���� |
| C����������CuSO4��Һ��Ӧʱ�����г������������������ݳ� |
| D�������ˮ��Ӧʮ�־��Ҳ�����H2��蘆��������ֱ����ˮ��Ӧ����Cs��OH��2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��1����֪���������Ȼ�ѧ����ʽ��
��1����֪���������Ȼ�ѧ����ʽ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| t/s | 0 | 120 | 180 | 240 | 330 | 530 | 600 | 700 | 800 |
| ��/% | 0 | 33.0 | 41.8 | 48.8 | 58.0 | 69.0 | 70.4 | 71.0 | 71.0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
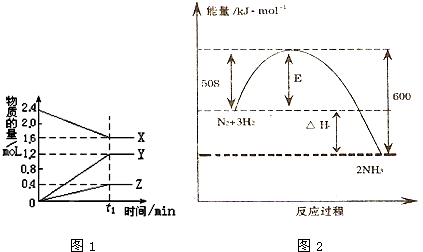
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���ɱ������˹����� |
| B��ˮ���������� |
| C���Ȼ�������ˮ |
| D�����������ۻ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com