
��10�֣��۲�����A��B��C��D��E�������ӣ�ԭ�ӻ����ӣ��Ľṹʾ��ͼ���ش��й����⣺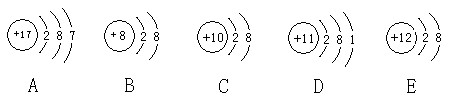
��1���������ӽṹʾ��ͼ���� �� �� �� ����д�����ţ���
��2�����������У��������ȶ���ԭ���� �� ������������д���ڱ�С������ͬ����������ʧȥ���ӵ�ԭ���� �������õ����ӵ�ԭ���� ��
��3��A��E����Ԫ���γɵĻ�������ˮ��Һ�еĵ��뷽��ʽ������������ ����
��4���ں˵����Ϊ1-18��Ԫ���У�д��������B������Ӳ��Ų���ͬ�����ӣ������ӵķ���Ϊ �� �� �� ��A-E���ѳ��ֵ�Ԫ�س��⣩��
��5����һ�������£�D���Ժ͵�����N2�����ϳ�һ�ְ�ɫ���ʣ������ʵĻ�ѧʽ������������ ����
 ���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
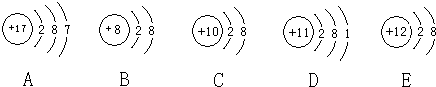
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
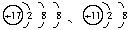
(1)���������Ӧ��Ԫ�ط�����_________����ԭ�����Ӧ�����ӵĽṹʾ��ͼ��_________��
(2)���Ӳ�ṹ��ͬ����_________(��д���ţ���ͬ)���������ȶ�����_________��������ʧȥ���ӵ���_________�������õ����ӵ���_________��
(3)��ֱ�������γɵĻ�����Ļ�ѧʽ��_________���ɾ�����ʧ���Ӻ��������γɵĻ�����Ļ�ѧʽ��_________��
(4)�ں˵����1��10��Ԫ���ڣ��о�������B���Ӳ�ṹ��ͬ�����ӣ�д�����ӵķ���_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ����������к����ؽ����ָ�һ��ѧ�����л�ѧ�Ծ� ���������� ���ͣ������
�۲�����A��B��C��D��E�������ӣ�ԭ�ӻ����ӣ��Ľṹʾ��ͼ���ش��й����⣺
��С��1���������ӽṹʾ��ͼ���� �� �� �� ����д�����ţ���
��С��2�����������У��������ȶ���ԭ���� �� ������������д���ڱ�С������ͬ����������ʧȥ���ӵ�ԭ���� �������õ����ӵ�ԭ���� ��
��С��3��A��E����Ԫ���γɵĻ�������ˮ��Һ�еĵ��뷽��ʽ�� ����
��С��4���ں˵����Ϊ1-18��Ԫ���У�д��������B������Ӳ��Ų���ͬ�����ӣ������ӵķ���Ϊ �� �� �� ��A-E���ѳ��ֵ�Ԫ�س��⣩��
��С��5����һ�������£�D���Ժ͵�����N2�����ϳ�һ�ְ�ɫ���ʣ������ʵĻ�ѧʽ������ ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com