
��֪�Ҷ���(HOOC�DCOOH���ɼ�дΪH2C2O4)�׳Ʋ��ᣬ������ˮ�����ڶ�Ԫ��ǿ��(Ϊ�������)��������ǿ��̼�ᣬ���۵�Ϊ101.5�棬��157��������ijУ�о���ѧϰС��Ϊ̽������IJ��ֻ�ѧ���ʣ�����������ʵ�飺
(1)��ʢ��1 mL����NaHCO3��Һ���Թ��м��������Ҷ�����Һ���۲쵽����ɫ���ݲ������÷�Ӧ�����ӷ���ʽΪ_________________________________________��
(2)��ʢ�������Ҷ��ᱥ����Һ���Թ��е����������ữ��KMnO4��Һ������������Һ���Ϻ�ɫ��ȥ��˵���Ҷ������_________________(������ԡ�������ԭ�ԡ������ԡ�)������ƽ�÷�Ӧ�����ӷ���ʽ��
____ MnO4�C + ____ H2C2O4 + _____ H+ = _____ Mn2+ + _____ CO2�� + _____ H2O
(3)��һ�������Ҷ�������Թ��У�����ͼ��ʾװ�ý���ʵ��(�г�װ��δ���)��
ʵ�鷢�֣�װ��C��G�г���ʯ��ˮ����ǣ�B��CuSO4��ĩ������F��CuO��ĩ��죬
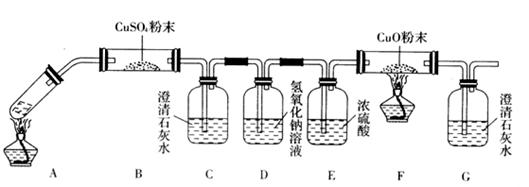
�ݴˣ��Ҷ���ֽ�IJ���Ϊ___________________________������װ���У�D��������_____
__________________________________________��װ��F�з�����Ӧ�Ļ�ѧ����ʽΪ��
___________________________________________________________��
(4)��С��ͬѧ��2.52 g���ᾧ��(H2C2O4??2H2O)���뵽100 mL 0.2 mol/L��NaOH��Һ�г�ַ�Ӧ����÷�Ӧ����Һ�����ԣ���ԭ����______________________
________________________________________________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪�Ҷ���(HOOC�DCOOH���ɼ�дΪH2C2O4)�׳Ʋ��ᣬ������ˮ�����ڶ�Ԫ��ǿ��(Ϊ�������)��������ǿ��̼�ᣬ���۵�Ϊ101.5�棬��157��������ijУ�о���ѧϰС��Ϊ̽������IJ��ֻ�ѧ���ʣ�����������ʵ�飺
(1)��ʢ��1 mL����NaHCO3��Һ���Թ��м��������Ҷ�����Һ���۲쵽����ɫ���ݲ������÷�Ӧ�����ӷ���ʽΪ_________________________________________________��
(2)��ʢ�������Ҷ��ᱥ����Һ���Թ��е����������ữ��KMnO4��Һ������������Һ���Ϻ�ɫ��ȥ��˵���Ҷ������_________________(������ԡ�������ԭ�ԡ������ԡ�)������ƽ�÷�Ӧ�����ӷ���ʽ��
____ MnO4�C + ____ H2C2O4 + _____ H+ = _____ Mn2+ + _____ CO2�� + _____ H2O
(3)��һ�������Ҷ�������Թ��У�����ͼ��ʾװ�ý���ʵ��(�г�װ��δ���)��
ʵ�鷢�֣�װ��C��G�г���ʯ��ˮ����ǣ�B��CuSO4��ĩ������F��CuO��ĩ��죬

�ݴˣ��Ҷ���ֽ�IJ���Ϊ______________________������װ���У�D��������______��װ��F�з�����Ӧ�Ļ�ѧ����ʽΪ�� _________________________________________��
(4)��С��ͬѧ��2.52g���ᾧ��(H2C2O4??2H2O)���뵽100 mL 0.2 mol/L��NaOH��Һ�г�ַ�Ӧ����÷�Ӧ����Һ�����ԣ���ԭ����_________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪�Ҷ���(HOOC�DCOOH���ɼ�дΪH2C2O4)�׳Ʋ��ᣬ������ˮ�����ڶ�Ԫ��ǿ��(Ϊ�������)��������ǿ��̼�ᣬ���۵�Ϊ101.5�棬��157��������ijУ�о���ѧϰС��Ϊ̽������IJ��ֻ�ѧ���ʣ�����������ʵ�飺
(1)��ʢ��1 mL����NaHCO3��Һ���Թ��м��������Ҷ�����Һ���۲쵽����ɫ���ݲ������÷�Ӧ�����ӷ���ʽΪ_________________________________________��
(2)��ʢ�������Ҷ��ᱥ����Һ���Թ��е����������ữ��KMnO4��Һ������������Һ���Ϻ�ɫ��ȥ��˵���Ҷ������_________________(������ԡ�������ԭ�ԡ������ԡ�)������ƽ�÷�Ӧ�����ӷ���ʽ��
____ MnO4�C + ____ H2C2O4 + _____ H+ = _____ Mn2+ + _____ CO2�� + _____ H2O
(3)��һ�������Ҷ�������Թ��У�����ͼ��ʾװ�ý���ʵ��(�г�װ��δ���)��
ʵ�鷢�֣�װ��C��G�г���ʯ��ˮ����ǣ�B��CuSO4��ĩ������F��CuO��ĩ��죬
�ݴˣ��Ҷ���ֽ�IJ���Ϊ___________________________������װ���У�D��������_____
__________________________________________��װ��F�з�����Ӧ�Ļ�ѧ����ʽΪ��
___________________________________________________________��
(4)��С��ͬѧ��2.52 g���ᾧ��(H2C2O4•2H2O)���뵽100 mL 0.2 mol/L��NaOH��Һ�г�ַ�Ӧ����÷�Ӧ����Һ�����ԣ���ԭ����______________________
________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011���Ĵ�ʡ�ɶ����и���12���¿������ۣ���ѧ���� ���ͣ�ʵ����
��֪�Ҷ���(HO OC�DCOOH���ɼ�дΪH2C2O4)�׳Ʋ��ᣬ������ˮ�����ڶ�Ԫ��ǿ��(Ϊ�������)��������ǿ��̼�ᣬ���۵�Ϊ101.5�棬��157��������ijУ�о���ѧϰС��Ϊ̽������IJ��ֻ�ѧ���ʣ�����������ʵ�飺
OC�DCOOH���ɼ�дΪH2C2O4)�׳Ʋ��ᣬ������ˮ�����ڶ�Ԫ��ǿ��(Ϊ�������)��������ǿ��̼�ᣬ���۵�Ϊ101.5�棬��157��������ijУ�о���ѧϰС��Ϊ̽������IJ��ֻ�ѧ���ʣ�����������ʵ�飺
(1)��ʢ��1 mL����Na HCO3��Һ���Թ��м��������Ҷ�����Һ���۲쵽����ɫ���ݲ������÷�Ӧ�����ӷ���ʽΪ_______
HCO3��Һ���Թ��м��������Ҷ�����Һ���۲쵽����ɫ���ݲ������÷�Ӧ�����ӷ���ʽΪ_______ __________________________________��
__________________________________��
(2)��ʢ�������Ҷ��ᱥ����Һ���Թ��е����������ữ��KMnO4��Һ������������Һ���Ϻ�ɫ��ȥ��˵���Ҷ������_________________(������ԡ�������ԭ�ԡ������ԡ�)������ƽ�÷�Ӧ�����ӷ���ʽ��
____ MnO4�C + ____ H2C2O4 + _____ H+ =" _____" Mn2+ + _____ CO2�� + _____ H2O
(3)��һ�������Ҷ�������Թ��У�����ͼ��ʾװ�ý���ʵ��(�г�װ��δ���)��
ʵ�鷢�֣�װ��C��G�г���ʯ��ˮ����ǣ�B��CuSO4��ĩ������F��CuO��ĩ��죬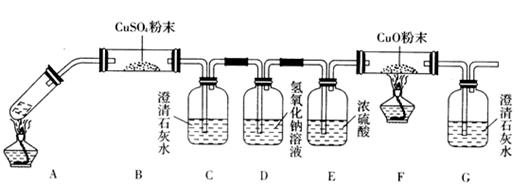
�ݴˣ��Ҷ���ֽ�IJ���Ϊ___________________________������װ���У�D��������_____
__________________________________________��װ��F�з�����Ӧ�Ļ�ѧ����ʽΪ��
___________________________________________________________��
(4)��С��ͬѧ��2.52 g���ᾧ��(H2C2O4?2H2O)���뵽100 mL 0.2 mol/L��NaOH��Һ�г�ַ�Ӧ����÷�Ӧ����Һ�����ԣ���ԭ����______________________
________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ���Ĵ�ʡ����12���¿������ۣ���ѧ���� ���ͣ�ʵ����
��֪�Ҷ���(HOOC�DCOOH���ɼ�дΪH2C2O4)�׳Ʋ��ᣬ������ˮ�����ڶ�Ԫ��ǿ��(Ϊ�������)��������ǿ��̼�ᣬ���۵�Ϊ101.5�棬��157��������ijУ�о���ѧϰС��Ϊ̽������IJ��ֻ�ѧ���ʣ�����������ʵ�飺
(1)��ʢ��1 mL����NaHCO3��Һ���Թ��м��������Ҷ�����Һ���۲쵽����ɫ���ݲ������÷�Ӧ�����ӷ���ʽΪ_________________________________________��
(2)��ʢ�������Ҷ��ᱥ����Һ���Թ��е����������ữ��KMnO4��Һ������������Һ���Ϻ�ɫ��ȥ��˵���Ҷ������_________________(������ԡ�������ԭ�ԡ������ԡ�)������ƽ�÷�Ӧ�����ӷ���ʽ��
____ MnO4�C + ____ H2C2O4 + _____ H+ = _____ Mn2+ + _____ CO2�� + _____ H2O
(3)��һ�������Ҷ�������Թ��У�����ͼ��ʾװ�ý���ʵ��(�г�װ��δ���)��
ʵ�鷢�֣�װ��C��G�г���ʯ��ˮ����ǣ�B��CuSO4��ĩ������F��CuO��ĩ��죬
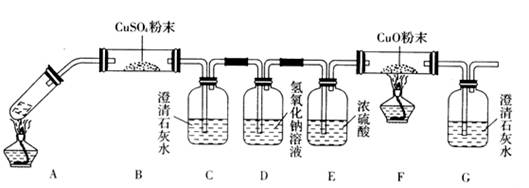
�ݴˣ��Ҷ���ֽ�IJ���Ϊ___________________________������װ���У�D��������_____
__________________________________________��װ��F�з�����Ӧ�Ļ�ѧ����ʽΪ��
___________________________________________________________��
(4)��С��ͬѧ��2.52 g���ᾧ��(H2C2O4•2H2O)���뵽100 mL 0.2 mol/L��NaOH��Һ�г�ַ�Ӧ����÷�Ӧ����Һ�����ԣ���ԭ����______________________
________________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com