
��ѧ�����ڻ�ѧ��ռ����Ҫ��λ����ش��������⣺
��1����״����6.72 L NH3����������ԭ������ mL H2O����ԭ������ȡ�
��2����֪16 g A��20 g Bǡ����ȫ��Ӧ����0��04 mol C��31��76 g D����C��Ħ������Ϊ ��
��3����V L����MgSO4��K2SO4�Ļ����Һ�ֳ����ȷݣ�һ�ݼ��뺬a mol NaOH����Һ��ǡ��ʹþ������ȫ����ΪMg(OH)2����һ�ݼ��뺬b mol BaCl2����Һ��ǡ��ʹSO42-��ȫ����ΪBaSO4����ԭ�����Һ�м����ӵ����ʵ���Ũ��Ϊ________������a��b��V��ʾ��
(1) 7.2 (2) 106 g/moL (3) 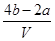 moL/L
moL/L
��������
�����������1������n��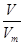 ��֪���ڱ�״����6.72 L NH3�����ʵ�����6.72L��22.4L/mol��0.3mol
��֪���ڱ�״����6.72 L NH3�����ʵ�����6.72L��22.4L/mol��0.3mol
�����к���ԭ�ӵ����ʵ�����0.3mol��4��1.2mol
����ˮ�����к���3��ԭ�ӣ��������ˮ������ԭ�ӵ����ʵ�����1.2mol
��ˮ�����ʵ�����1.2mol��3��0.4mol
��������0.4mol��18g/mol��7.2g
����ˮ�������7.2ml
��2��16 g A��20 g Bǡ����ȫ��Ӧ����0��04 mol C��31��76 g D
����������غ㶨�ɿ�֪������C��������16g��20g��31.76g��4.24g
����C��Ħ��������4.24g��0.04mol��106g/mol
��3��һ�ݼ��뺬a mol NaOH����Һ��ǡ��ʹþ������ȫ����ΪMg(OH)2������ݷ���ʽ��֪
Mg2����2OH����Mg(OH)2��
1mol 2mol
0.5amol amol
��һ�ݼ��뺬b mol BaCl2����Һ��ǡ��ʹSO42-��ȫ����ΪBaSO4������ݷ���ʽ��֪
Ba2����SO42����BaSO4��
1mol 1mol
bmol bmol
���ԭ��Һ��Mg2����SO42�������ʵ����ֱ���amol��2bmol
�������Һ�ĵ����Կ�֪��ԭ�����Һ�м����ӵ����ʵ�����4bmol��2amol
����ԭ�����Һ�м����ӵ����ʵ���Ũ�ȣ�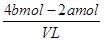 ��
��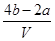 mol/L
mol/L
���㣺�������ʵ���������Ħ�������Ħ�������Լ����ʵ���Ũ�ȵ��йؼ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧ�����ڻ�ѧ��ռ����Ҫ��λ����ش��������⣺
��1������mgij���壬����˫ԭ�ӷ��ӹ��ɣ���Ħ������ΪMg��mol-1���������ӵ�������NA��ʾ����
�ٸ���������ʵ���Ϊ________��
�ڸ���������ԭ������Ϊ ����
�۸������ڱ�״���µ����Ϊ__________��
��2�����״����V LCO2������ԭ����Ŀ��ͬ��ˮ��������_______���÷�ʽ��ʾ����
��3����һ�����¶Ⱥ�ѹǿ�£�1�������X2��3�������Y2��������2����������û�����Ļ�ѧʽ��______________��
��4��ij�λ����Һ�к������ӣ�Na+��Mg2+ ��Cl-��SO42-�����Na+��Mg2+ ��Cl-
�����ʵ���Ũ������Ϊ�� 0.2mol��L-1��0.25 mol��L-1��0.4mol��L-1����c��SO42-��=___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��8�֣���ѧ�����ڻ�ѧ��ռ����Ҫ��λ����ش��������⣺
��1������mgij���壬����˫ԭ�ӷ��ӹ��ɣ���Ħ������ΪMg��mol-1���������ӵ�������NA��ʾ������������ԭ������Ϊ ����
��2�����״����V LCO2������ԭ����Ŀ��ͬ��ˮ��������_______���÷�ʽ��ʾ����
��3����һ�����¶Ⱥ�ѹǿ�£�1�������X2��3�������Y2��������2����������û�����Ļ�ѧʽ��______________��
��4��ij�λ����Һ�к������ӣ�Na+��Mg2+ ��Cl-��SO42-�����Na+��Mg2+ ��Cl-
�����ʵ���Ũ������Ϊ�� 0.2 mol��L-1��0.25 mol��L-1��0.4 mol��L-1����c��SO42-��=___________ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012��ɽ��ʡΫ�������ظ�һ��ѧ��ģ��ѧ���϶���⻯ѧ�Ծ� ���ͣ������
��ѧ�����ڻ�ѧ��ռ����Ҫ��λ����ش��������⣺
��1������mgij���壬����˫ԭ�ӷ��ӹ��ɣ���Ħ������ΪMg��mol-1���������ӵ�������NA��ʾ����
�ٸ���������ʵ���Ϊ___ _____��
_____��
�ڸ���������ԭ������Ϊ ����
�۸������ڱ�״���µ����Ϊ__________��
��2�����״����V LCO2������ԭ����Ŀ��ͬ��ˮ��������_______���÷�ʽ��ʾ����
��3����һ�����¶Ⱥ�ѹǿ�£�1�������X2��3�������Y2��������2����������û�����Ļ�ѧʽ��______________��
��4��ij�λ����Һ�к������ӣ�Na+��Mg2+��Cl-��SO42 -�����Na+��Mg2
-�����Na+��Mg2 +��Cl-
+��Cl-
�����ʵ���Ũ������Ϊ��0.2 mol��L-1��0.25 mol��L-1��0.4 mol��L-1����c��SO42-��="___________" ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��ɽ��ʡ��һ12��ѧ����п��Ի�ѧ�Ծ� ���ͣ������
��8�֣���ѧ�����ڻ�ѧ��ռ����Ҫ��λ����ش��������⣺
��1������mgij���壬����˫ԭ�ӷ��ӹ��ɣ���Ħ������ΪMg��mol-1���������ӵ�������NA��ʾ������������ԭ������Ϊ ����
��2�����״����V LCO2������ԭ����Ŀ��ͬ��ˮ��������_______���÷�ʽ��ʾ����
��3����һ�����¶Ⱥ�ѹǿ�£�1�������X2��3�������Y2��������2����������û�����Ļ�ѧʽ��______________��
��4��ij�λ����Һ�к������ӣ�Na+��Mg2+ ��Cl-��SO42-�����Na+��Mg2+ ��Cl-
�����ʵ���Ũ������Ϊ�� 0.2 mol��L-1��0.25 mol��L-1��0.4 mol��L-1����c��SO42-��=___________ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com