
����Ŀ��A��B��C��D��E���Ƕ�����Ԫ�أ�ԭ��������������A��B����ͬһ���ڣ�C��D��Eͬ����һ���ڡ�C��B�ɰ�ԭ�Ӹ�����2:1��1:1�ֱ��γ��������ӻ�������ҡ�D��A��ԭ�Ӹ�����3:2�γ����ӻ��������E�ǵؿ��к�����ߵĽ���Ԫ�ء�����������Ϣ�ش���������:
��1��BԪ�������ڱ��е�λ����_____________�������ʵĵ���ʽ��______________________��
��2��A��B��C��D��E����Ԫ�ص�ԭ�Ӱ뾶��С�����˳����____________(��Ԫ�ط�����д)
��3��E�ĵ�����C������������Ӧ��ˮ�������Һ������Ӧ�����ӷ���ʽ��______________________________________��
��4��Ԫ��A��ԭ�Ӻ����_____����״��ͬ�ĵ����ơ�
��5������Ԫ��C���ε���ɫ��ӦΪ________________ɫ����������ζ����Է�����ɫ��Ӧ����ԭ����________________________________________________________��
���𰸡� ������ VIA�� ![]() Na>Mg>Al>N>O 2Al+2OH-+2H2O=2AlO2-+3H2�� 2 �� ����̬�ĵ��Ӵ������ߵĹ��ԾǨ�������͵Ĺ��ʱ,��һ���IJ���(�ɼ�������)�����ʽ�ͷ�����
Na>Mg>Al>N>O 2Al+2OH-+2H2O=2AlO2-+3H2�� 2 �� ����̬�ĵ��Ӵ������ߵĹ��ԾǨ�������͵Ĺ��ʱ,��һ���IJ���(�ɼ�������)�����ʽ�ͷ�����
�����������������A��B��C��D��E���Ƕ�����Ԫ�أ�ԭ��������������A��B����ͬһ���ڣ�C��D��Eͬ����һ���ڡ�C��B�ɰ�ԭ�Ӹ�����2:1��1:1�ֱ��γ��������ӻ������������BΪNa��CΪO����Ϊ�����ơ���Ϊ����������D��A��ԭ�Ӹ�����3:2�γ����ӻ����������AΪN��DΪMg����Ϊ��������þ��E�ǵؿ��к�����ߵĽ���Ԫ������EΪAl��
��1��BԪ�������ڱ��е�λ���Ƕ����� VIA�壬�����ʵĵ���ʽ��![]() ��
��
��2��A��B��C��D��E����Ԫ�ص�ԭ�Ӱ뾶��С�����˳����Na>Mg>Al>N>O ��
��3��E�ĵ�����C������������Ӧ��ˮ�������Һ������Ӧ�����ӷ���ʽ��2Al+2OH-+2H2O=2AlO2-+3H2����
��4��Ԫ��A�Ļ�̬ԭ�ӵĵ����Ų�ʽΪ1s22s22p3��s��������εġ�p����ǷĴ��εģ�������ԭ�Ӻ����2����״��ͬ�ĵ����ơ�
��5������Ԫ��C���ε���ɫ��ӦΪ��ɫ����������ζ����Է�����ɫ��Ӧ����ԭ����������̬�ĵ��Ӵ������ߵĹ��ԾǨ�������͵Ĺ��ʱ,��һ���IJ���(�ɼ�������)�Ĺ����ʽ�ͷ�������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧ������װ���Ʊ�������Cl2�����ʡ�����˵����ȷ����(�� ��)
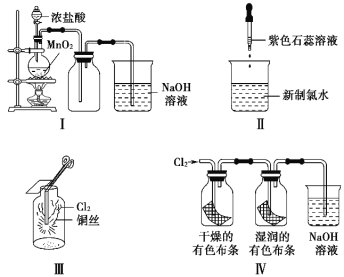
A. ��ͼ����MnO2��������Ũ�����ȫ��������
B. ��ͼ��֤��������ˮֻ������
C. ��ͼ���������ػ�ɫ����
D. ��ͼ��ʪ�����ɫ������ɫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��þ��CO2�����϶�Ŀ�����ȼ�յIJ�����
A. MgO B. MgO��C C. MgO��Mg3N2 D. MgO��Mg3N2 ��C
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ClO2��һ��ɱ������Ч�ʸߡ�������ȾС��ˮ��������ʵ�����п�ͨ�����·�Ӧ�Ƶã�2KClO3��H2C2O4��H2SO4![]() 2ClO2����K2SO4��2CO2����2H2O������˵������ȷ������ ��
2ClO2����K2SO4��2CO2����2H2O������˵������ȷ������ ��
A��CO2����������
B��H2C2O4�ڷ�Ӧ�б�����
C��H2C2O4��������ǿ��ClO2��������
D��ClO2��ˮ������ʱ����������ǿ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������������������У��ı����������ܶ࣬������������ء���ù�ء����������17�������ص�ı���������硣
��1����ԭ���ڻ�̬ʱ���۵����Ų�ʽΪ__________________________________��
��2�� �������NO3-�Ŀռ乹��Ϊ________��д����NO3-��Ϊ�ȵ������һ�ַǼ��Է��ӻ�ѧʽ_________________________________��
��3��6-������ù����Ľṹ��ͼ��ʾ�����в���sp3�ӻ���ԭ����___________��

��4����ͼ����ʾΪѪ���صĽṹ��Ѫ���������ַǽ���Ԫ�صĵ縺����С�����˳����_______����ͼ�������ʵ��ķ�ʽ�����λ��________��

��5���ڶ������辧���У���ԭ�ӵļ۵��Ӳ�ԭ�ӹ���������ӻ����ӻ��ķ�ʽ��______��O-Si-O �н���____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ��������ͼ��ʾװ���Ʊ�����������һϵ�����ʵ��(�г��豸����Ҫ�ļ���װ����ʡ��)��

(1)����װ��װ����Ϻ����Ƚ��еIJ�����______________________���������Լ���
(2)ʵ���ҳ���MnO2+4HCl(Ũ)![]() MnCl2+Cl2��+2H2O��ȡ������
MnCl2+Cl2��+2H2O��ȡ������
��ʵ��ʱ��ͨ�������ܶ�Ϊ1��19g/cm3��Ũ��Ϊ36��5%��Ũ���ᡣ��Ũ��������ʵ���Ũ��Ϊ___________________________��
�ڱ�״���£�������Ӧÿ����4.48L������ת�Ƶ��ӵ����ʵ���Ϊ_________ mol��
(3)ϴ��װ��B�������dz�ȥCl2�е�HCl���壬ͬʱ������ȫƿ(���ʵ��ʱװ��C���Ƿ�������)����װ��C����������װ��B�н��۲쵽��������_____________��
(4)װ��C����������֤�����Ƿ����Ư���ԡ�Ϊ�ˣ�ʵ��ʱװ��C��I��II��III�����η����������___________(����ĸ)��
A | B | C | D | |
I | �������ɫ���� | ʪ�����ɫ���� | �������ɫ���� | ʪ�����ɫ���� |
II | ��ʯ�� | ��ˮ�Ȼ��� | ��ˮ�Ȼ��� | Ũ���� |
III | ʪ�����ɫ���� | �������ɫ���� | ʪ�����ɫ���� | �������ɫ���� |
(5)���װ��D��E��Ŀ���DZȽ��ȡ��塢��������ԡ�����D�л���ͨ��һ��������ʱ�����Կ�����ɫ��Һ��Ϊ��ɫ��˵�������������Ա��嵥�ʵ�______(����ǿ������������)����������װ��D��������Һ����װ��E�У����ã��۲쵽��������___________��
(6)FΪβ������װ�á�ʵ��ʱ����װ���з�Ӧ�����ӷ���ʽ______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ�̶��ݻ����ܱ������г�������A��B���������¿��淴Ӧ��A(g) +B(g)![]() xC(g)����H �� Q���ڲ�ͬ���¶Ⱥ�ѹǿ�£�C�ڷ�Ӧ������еĺ����淴Ӧʱ��ı仯��ͼ��ʾ�������ж���ȷ����
xC(g)����H �� Q���ڲ�ͬ���¶Ⱥ�ѹǿ�£�C�ڷ�Ӧ������еĺ����淴Ӧʱ��ı仯��ͼ��ʾ�������ж���ȷ����
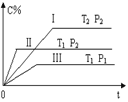
A. P1��P2��x��1 B. P1��P2��x��1
C. T1��T2��Q��0 D. T1��T2��Q��0
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ʵ����ͺ���Ԫ�صĻ��ϼ����о��������ʵ�������Ҫ�Ƕȡ���ͼ��ʾ�����䲿�ֻ�������������άͼ���� ����ͼʾ�ش��������⣺

(1)ͼ��A��B��C��D���������У����ڷǵ���ʵ���__________(�û�ѧʽ��ʾ)��
(2)����Bͨ����ɫʯ����Һ��������__________________________��
��һ����ѧ����ʽ˵��B���л�ԭ��_____________________________��
(3)C�ڷ�Ӧ�мȿ������������ֿ�����ԭ����������������ʱ����������ɱ���ԭΪ__________(����)��
A��Na2S B��S C��H2SO3 D��Na2SO4 E��H2SO4
(4)��A��SO2��ϣ������ɵ���ɫ���塣�÷�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ_____��
(5)D��Ũ��Һ��ͭ�����ڼ��������¿��Է�����ѧ��Ӧ����Ӧ�Ļ�ѧ����ʽΪ_________________����Ӧ��D��ʾ���Ժ�______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ�е�ÿһ�����ʾ�йص�һ�ַ�Ӧ�����������мס��ҡ�������Ϊ�������ʣ������Ϊ�����A��һ�ֳ�����Һ̬�����B�Ǿ��д��Ե������D�Ͷ����ܺ��ᷴӦ���ܺͼӦ�����ڱ���ȼ�ղ�����ɫ���棬H�ڿ����к����ױ�������I�����ǵ�ת����ϵ���£�����Щ��Ӧ�������Ͳ��ֲ���δע����

��1��д���������ʻ�ѧʽ��B____________��F____________��
��2��д����Ӧ�ٵĻ�ѧ����ʽ��________________________________________��
��3��H�ڿ����к����ױ�������I���ù��̵�ʵ��������_______________________________��
��4����������CO2ͨ��E��Һ�У����ӷ���ʽ��______________________________________��
��5����G�ı�����Һ�����ˮ�еú��ɫҺ�壬��Һ����е�������_______��
A�����ж����ЧӦ B�����ɫҺ����ù��˵ķ����ᴿ
C��Һ���з�ɢ����ֱ��С��1nm D��ȡ����Һ�����K2SO4��Һ��������ɫ���� E. �����ȶ��Ĵ��ڣ�����������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com