
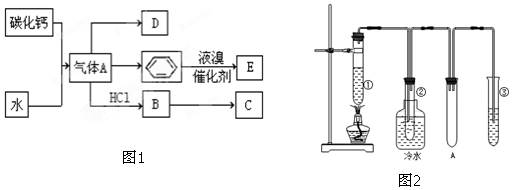
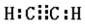 ��
�� ���䷴Ӧ����Ϊ�Ӿ۷�Ӧ��
���䷴Ӧ����Ϊ�Ӿ۷�Ӧ��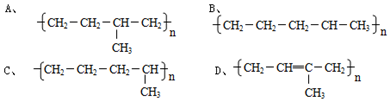
���� ̼������ˮ��Ӧ����AΪ��Ȳ��D��һ��ֲ���������ڼ����������Դ����ʵ����DΪ��ϩ����Ȳ�����������ӳɷ�Ӧ������ϩ����Ȳ��HCl�����ӳɷ�Ӧ����B��B������Ӧ���ɺϳ���֬C����BΪCH2=CHCl��CΪ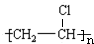 ������Һ���ڴ��������·���ȡ����Ӧ����EΪ
������Һ���ڴ��������·���ȡ����Ӧ����EΪ ��
��
���Թܢ��м���ʯ���ͺ�����������ʯ���ֽ⣩�������ѻ���Ӧ����ϩ�����Թܢڷ�����ˮ�У����ڷ���Һ�������壬�Թܢ��м�����ˮ�������ڼ���ϩ�����Թ�A���ڷ��������Դ˽����⣮
��� �⣺��1���������Ϸ�����AΪ��Ȳ������ʽΪ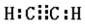 ���ʴ�Ϊ��
���ʴ�Ϊ��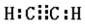 ��
��
��2��������Ȳ�Ļ�ѧ����ʽΪ��CaC2+2H2O��Ca��OH��2+HC��CH����B��C������ϩ�����Ӿ۷�Ӧ���ɾ�����ϩ����Ӧ��ѧ����ʽΪ��nCH2=CHCl$\stackrel{����}{��}$ ��
��
�ʴ�Ϊ��CaC2+2H2O��Ca��OH��2+HC��CH����nCH2=CHCl$\stackrel{����}{��}$ ���Ӿ۷�Ӧ��
���Ӿ۷�Ӧ��
��4��CH2=CH-CH3��CH2=CH2������ˮ�����ӳɷ�Ӧ��ʹ��ѹ���ͣ�����������װ��A�������Ƿ�ֹ�Թܢ���Һ�嵹�����Թܢ��У�
�ʴ�Ϊ����ֹ�Թܢ���Һ�嵹�����Թܢ��У���������ȫƿ����
��5������������ѽⷽʽΪ��C4H10��CH4+CH2=CHCH3��C4H10��C2H6+CH2=CH2���������ϩ�ͱ�ϩ֮�䷢���Ӿ۷�Ӧ������̼ԭ��֮�������˳������� ��
��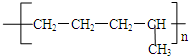 ���ֽṹ��ʽ���ֱ�ΪA��C�ṹ��
���ֽṹ��ʽ���ֱ�ΪA��C�ṹ��
�ʴ�Ϊ��CH2=CH-CH3��CH2=CH2��AC��
��6��CH2=CH-CH3��CH2=CH2������ˮ�����ӳɷ�Ӧ������ʽΪCH2=CH2+Br2��CH2BrCH2Br��CH3CH=CH2+Br2��CH3CHBrCH2Br����
�ʴ�Ϊ��CH2=CH2+Br2��CH2BrCH2Br��CH3CH=CH2+Br2��CH3CHBrCH2Br����
���� ���⿼���л���ϳɣ�Ϊ��Ƶ���㣬���ؿ���ѧ���ķ���������ʵ�������Ŀ��飬ע�����A��������ת��Ϊ��ϩ���������ƶϣ���Ŀ������ǿ�������̲ģ�ע��Ի���֪ʶ���������գ�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ʹ����ˮ����ֲ���ͺ����� | |
| B�� | ���ö�����̼����ȫ�������ϣ�������������ЧӦ | |
| C�� | �����úš������ϵ�̫���ܵ�صIJ����ǹ� | |
| D�� | ʵ���ҽ�����ʧ��ʱ����ʹ����ĭ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
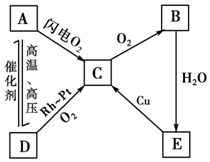 ��ͼ��ʾ����A��B��C��D��E���ֺ������ʵ�ת����ϵͼ������A��B��C��D�����¶������壬BΪ����ɫ��д��A��B��C��D��E�Ļ�ѧʽ������Ӧ�Ļ�ѧ����ʽ��
��ͼ��ʾ����A��B��C��D��E���ֺ������ʵ�ת����ϵͼ������A��B��C��D�����¶������壬BΪ����ɫ��д��A��B��C��D��E�Ļ�ѧʽ������Ӧ�Ļ�ѧ����ʽ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
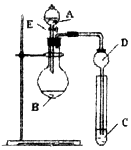 ij�о���ѧϰС�������һ��ʵ��̽��Ԫ�������ɣ���ͬѧ����Ԫ�طǽ��������Ӧ��ۺ�����֮��Ĺ�ϵ���������ͼװ��һ�������N��C��Si�ķǽ�����ǿ���Ƚϵ�ʵ���о�����ͬѧ�����û���Ӧ�Ĺ��ɣ�����ͼװ�������OԪ�صķǽ����Ա�Sǿ��ʵ���о����ش��������⣺
ij�о���ѧϰС�������һ��ʵ��̽��Ԫ�������ɣ���ͬѧ����Ԫ�طǽ��������Ӧ��ۺ�����֮��Ĺ�ϵ���������ͼװ��һ�������N��C��Si�ķǽ�����ǿ���Ƚϵ�ʵ���о�����ͬѧ�����û���Ӧ�Ĺ��ɣ�����ͼװ�������OԪ�صķǽ����Ա�Sǿ��ʵ���о����ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | HCO3-+H2O?H3O++CO32- | B�� | ClO-+H3O+?HClO+H2O | ||
| C�� | HS-+H2O?H2S+OH- | D�� | NH4++OH-?NH3•H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
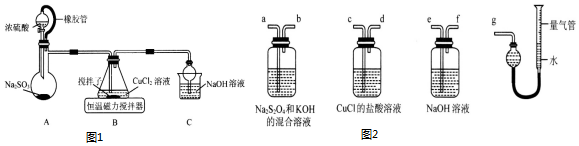
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1mol����ϩ�к��е�̼̼˫����Ϊ4NA | |
| B�� | ��״���£�22.4 L Cl2��ˮ��Ӧת�Ƶĵ�����ΪNA | |
| C�� | 50���£�1L pH=2��ϡ������Һ�к��е�H+��ĿΪ0.01NA | |
| D�� | 30 g��������������Ļ�����к��е�̼ԭ����ĿΪNA |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com