
ŌŚ£·£¶£³””KŗĶ£³£®£°£“”Į£±£°£“£ė£Š£įŹ±£¬ÓĆCOÓė£Č£²ŗĻ³É£Ć£Č£³£Ļ£Č£ŗ£Ć£Ļ£Ø£ē£©£«£²£Č£²£Ø£ē£©![]() £Ć£Č£³£Ļ£Č£Ø£ē£©”£
£Ć£Č£³£Ļ£Č£Ø£ē£©”£
(1)ÉčĘšŹ¼Ź±£¬£ī£Ø£Č£²£©”Ć£ī£ØCO£©£½£ķ£¬Ę½ŗāŹ±CO×Ŗ»ÆĀŹĪŖ¦Į£¬£Ć£Č£³£Ļ£ČµÄĢå»ż·ÖŹżĪŖ£ł£¬Ōņ£ķ”¢¦Į”¢£łČżÕߵĹŲĻµŹĒ___________”£
(2)½«ĻĀ±ķŅŃÖŖŹż¾Ż“śČėÉĻŹö¹ŲĻµ£¬½ųŠŠ¼ĘĖć£¬½«½į¹ūĢīČė±ķÖŠ£ŗ
| £ķ | ¦Į | £ł |
| 1 | 0.25 | ¢Ł |
| 2 | 0.45 | ¢Ś |
| 3 | ¢Ū | 19.35% |
(3)øł¾Ż±ķÖŠŹż¾Ż£¬æÉµĆ³ö×ī¼Ń·“Ó¦ĪļµÄÅä±Č£ķŹĒ_________£¬ŌŅņŹĒ______________”£
(1)y=![]() ”Į£±£°£°£„
”Į£±£°£°£„
(2)16.67% 21.43% 0.56
(3)2µ±m=2Ź±£¬Ę½ŗāĘųĢåÖŠ£Ć£Č£³£Ļ£ČµÄĢå»ż·ÖŹż×ī“ó
(1)ÉčæŖŹ¼Ź±COĪŖ£±””£ķ£ļ£ģ£¬Ōņ£Č£²ĪŖ£ķ””£ķ£ļ£ģ£¬
ÓÉ£ŗ CO£« £²£Č£²![]() £Ć£Č£³£Ļ£Č
£Ć£Č£³£Ļ£Č
£īŹ¼/£ķ£ļ£ģ£ŗ1 £ķ £°
£ī±ä/£ķ£ļ£ģ£ŗ¦Į £²¦Į ¦Į
£īĘ½/£ķ£ļ£ģ£ŗ£±£¦Į £ķ££²¦Į ¦Į
Ę½ŗāŹ±£ī(×Ü£©£½£Ø£±£«£ķ££²¦Į£©£ķ£ļ£ģ£¬
¹Ź£ł£½![]() ”Į£±£°£°£„”£
”Į£±£°£°£„”£
(2)Ö±½Ó“śČė(1)Ź½½ųŠŠ¼ĘĖćµĆ£ŗ£±£¶£®£¶£·£„”¢£²£±£®£“£³£„”¢£°£®£µ£¶”£
(3)ÓÉ(2)ÖŖµ±£ķ£½£²Ź±£¬ĪŖ×ī¼ŃÅä±Č”£


 ±øÕ½ÖŠæ¼ŗ®¼ŁĻµĮŠ“š°ø
±øÕ½ÖŠæ¼ŗ®¼ŁĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗŗŚĮś½Ź”Ė«Ń¼É½Ņ»ÖŠ2011£2012ѧğø߶žÉĻŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌĢā ĢāŠĶ£ŗ022
| |||||||||||||||||||||||||||||||||||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖ»Æѧ·“Ó¦¢Ł£ŗFe(s)£«CO2(g) FeO(s)£«CO(g)£¬ĘäĘ½ŗā³£ŹżĪŖK1£»»Æѧ·“Ó¦¢Ś£ŗFe(s)£«H2O(g)
FeO(s)£«H2(g)£¬ĘäĘ½ŗā³£ŹżĪŖK2£¬ŌŚĪĀ¶Č973 KŗĶ1173KĒéæöĻĀ£¬K1”¢K2µÄÖµ·Ö±šČēĻĀ£ŗ
| ĪĀ¶Č | K1 | K2 |
| 973 K | 1.47 | 2.38 |
| 1173 K | 2.15 | 1.67 |
ĒėĢīæÕ£ŗ
(1)Ķعż±ķøńÖŠµÄŹżÖµæÉŅŌĶʶĻ£ŗ·“Ó¦¢ŁŹĒ________(Ģī”°ĪüČČ”±»ņ”°·ÅČČ”±)·“Ó¦£®
(2)ĻÖÓŠ·“Ó¦¢Ū£ŗCO2(g)£«H2(g) CO(g)£«H2O(g)£¬ĒėÄ抓³öøĆ·“Ó¦µÄĘ½ŗā³£ŹżK3µÄŹżŃ§±ķ“ļŹ½£ŗK3£½ .
(3)ŌŚĻąĶ¬ĪĀ¶ČĻĀ£¬øł¾Ż·“Ó¦¢ŁÓė¢ŚæÉĶʵ¼³öK1”¢K2ÓėK3Ö®¼äµÄ¹ŲĻµŹ½________£¬¾Ż“Ė¹ŲĻµŹ½¼°ÉĻ±ķŹż¾Ż£¬Ņ²ÄÜĶʶĻ³ö·“Ó¦¢ŪŹĒ________(Ģī”°ĪüČČ”±»ņ”°·ÅČČ”±)·“Ó¦£®
(4)ŅŖŹ¹·“Ó¦¢ŪŌŚŅ»¶ØĢõ¼žĻĀ½ØĮ¢µÄĘ½ŗāĻņÕż·“Ó¦·½ĻņŅĘ¶Æ£¬æɲÉČ”µÄ“ėŹ©ÓŠ________(ĢīŠ“×ÖÄøŠņŗÅ£¬ĻĀĶ¬)£®
A£®Ōö“óŃ¹Ēæ B£®Ģå»ż²»±äŹ±³äČėĻ”ÓŠĘųĢå C£®ÉżøßĪĀ¶Č
D£®Ź¹ÓĆŗĻŹŹµÄ“߻ƼĮ E£®ŌŚĢåĻµÖŠĶ¶ČėÉŁĮæP2O5¹ĢĢå
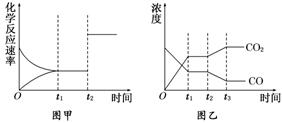
(5)Ķ¼¼×”¢ŅŅ·Ö±š±ķŹ¾·“Ó¦¢ŪŌŚt1Ź±æĢ“ļµ½Ę½ŗā£¬ŌŚt2Ź±æĢŅņøıäijøöĢõ¼ž¶ų·¢Éś±ä»ÆµÄĒéæö£ŗ¢ŁĶ¼¼×ÖŠt2Ź±æĢ·¢ÉśøıäµÄĢõ¼žæÉÄÜŹĒ________________£®
¢ŚĶ¼ŅŅÖŠt2Ź±æĢ·¢ÉśøıäµÄĢõ¼žæÉÄÜŹĒ £®
A.ÉżøßĪĀ¶Č B.½µµĶĪĀ¶Č C.¼ÓČė“߻ƼĮ D.Ōö“óŃ¹Ēæ
E.¼õŠ”Ń¹Ēæ F.³äČėCO2 G.·ÖĄė³ö²æ·ÖCO
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013ѧğ¹ć¶«·šÉ½ŹŠÖŠ“óø½ÖŠø߶žÉĻĘŚÖŠæ¼ŹŌĄķæĘ»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗĢīæÕĢā
ŅŃÖŖ»Æѧ·“Ó¦¢Ł£ŗFe£Øs£©+CO2£Øg£©=FeO£Øs£©+CO£Øg£©,ĘäĘ½ŗā³£ŹżĪŖK1£»
»Æѧ·“Ó¦¢Ś£ŗFe£Øs£©+H2O£Øg£©=FeO£Øs£©+H2£Øg£©,ĘäĘ½ŗā³£ŹżK2”£
ŌŚĪĀ¶Č973 KŗĶ1173 KĒéæöĻĀ£¬K1”¢K2µÄÖµ·Ö±šČēĻĀ£ŗ
| ĪĀ¶Č | K1 | K2 |
| 973 K | 1.47 | 2.38 |
| 1173 K | 2.15 | 1.67 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2014½ģŗÓ±±Ź”ø߶ž10ŌĀŌĀæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
£Ø9·Ö£©ŅŃÖŖ»Æѧ·“Ó¦¢Ł£ŗFe£Øs£©£«CO2£Øg£© FeO£Øs£©£«CO£Øg£©£¬ĘäĘ½ŗā³£ŹżĪŖK1£»»Æѧ·“Ó¦¢Ś£ŗFe£Øs£©£«H2O£Øg£©
FeO£Øs£©£«CO£Øg£©£¬ĘäĘ½ŗā³£ŹżĪŖK1£»»Æѧ·“Ó¦¢Ś£ŗFe£Øs£©£«H2O£Øg£© FeO£Øs£©£«H2£Øg£©£¬ĘäĘ½ŗā³£ŹżĪŖK2£¬ŌŚĪĀ¶Č973 KŗĶ1173 KĒéæöĻĀ£¬K1”¢K2µÄÖµ·Ö±šČēĻĀ£ŗ
FeO£Øs£©£«H2£Øg£©£¬ĘäĘ½ŗā³£ŹżĪŖK2£¬ŌŚĪĀ¶Č973 KŗĶ1173 KĒéæöĻĀ£¬K1”¢K2µÄÖµ·Ö±šČēĻĀ£ŗ
|
ĪĀ¶Č |
K1 |
K2 |
|
973 K |
1£®47 |
2£®38 |
|
1173 K |
2£®15 |
1£®67 |
ĒėĢīæÕ£ŗ
£Ø1£©Ķعż±ķøńÖŠµÄŹżÖµæÉŅŌĶʶĻ£ŗ·“Ó¦¢ŁŹĒ________£ØĢī”°ĪüČČ”±»ņ”°·ÅČČ”±£©·“Ó¦£®
£Ø2£©ĻÖÓŠ·“Ó¦¢Ū£ŗCO2£Øg£©£«H2£Øg£© ??CO£Øg£©£«H2O£Øg£©£¬ĒėÄ抓³öøĆ·“Ó¦µÄĘ½ŗā³£ŹżK3µÄŹżŃ§±ķ“ļŹ½£ŗK3£½________£®
??CO£Øg£©£«H2O£Øg£©£¬ĒėÄ抓³öøĆ·“Ó¦µÄĘ½ŗā³£ŹżK3µÄŹżŃ§±ķ“ļŹ½£ŗK3£½________£®
£Ø3£©øł¾Ż·“Ó¦¢ŁÓė¢ŚæÉĶʵ¼³öK1”¢K2ÓėK3Ö®¼äµÄ¹ŲĻµŹ½________£¬¾Ż“Ė¹ŲĻµŹ½¼°ÉĻ±ķŹż¾Ż£¬Ņ²ÄÜĶʶĻ³ö·“Ó¦¢ŪŹĒ________£ØĢī”°ĪüČČ”±»ņ”°·ÅČČ”±£©·“Ó¦£®
£Ø4£©ŅŖŹ¹·“Ó¦¢ŪŌŚŅ»¶ØĢõ¼žĻĀ½ØĮ¢µÄĘ½ŗāĻņÕż·“Ó¦·½ĻņŅĘ¶Æ£¬æɲÉČ”µÄ“ėŹ©ÓŠ________£ØĢīŠ“×ÖÄøŠņŗÅ£©£®
A£®ĖõŠ”·“ӦȯĘ÷µÄČŻ»ż
B£®Ą©“ó·“ӦȯĘ÷µÄČŻ»ż
C£®ÉżøßĪĀ¶Č
D£®Ź¹ÓĆŗĻŹŹµÄ“߻ƼĮ
E£®Éč·Ø¼õŠ”Ę½ŗāĢåĻµÖŠµÄCOµÄÅضČ
£Ø5£©Ķ¼¼×”¢ŅŅ·Ö±š±ķŹ¾·“Ó¦¢ŪŌŚt1Ź±æĢ“ļµ½Ę½ŗā£¬ŌŚt2Ź±æĢŅņøıäijøöĢõ¼ž¶ų·¢Éś±ä»ÆµÄĒéæö£ŗ
¢ŁĶ¼¼×ÖŠt2Ź±æĢ·¢ÉśøıäµÄĢõ¼žŹĒ_____________________________________£®
¢ŚĶ¼ŅŅÖŠt2Ź±æĢ·¢ÉśøıäµÄĢõ¼žŹĒ_______________________________________£®
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com