
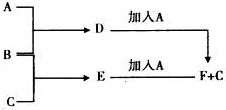
 A��B��CΪ3�ֵ��ʣ�����AΪ���壬B��CΪ���壩����D�ı�����Һ�����ˮ�м�����У���Һ�ʺ��ɫ��B��C��Ӧ�IJ���������ˮ�õ���ɫ��ҺE������֮���ת����ϵ��ͼ��
A��B��CΪ3�ֵ��ʣ�����AΪ���壬B��CΪ���壩����D�ı�����Һ�����ˮ�м�����У���Һ�ʺ��ɫ��B��C��Ӧ�IJ���������ˮ�õ���ɫ��ҺE������֮���ת����ϵ��ͼ��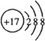 ��
�� ��
��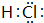 �������Ȼ�����Һ�����ˮ�м�����У����������������壬��Һ�ʺ��ɫ�����ӷ���ʽΪ��Fe3++3H2O
�������Ȼ�����Һ�����ˮ�м�����У����������������壬��Һ�ʺ��ɫ�����ӷ���ʽΪ��Fe3++3H2O
| ||
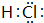 ��Fe3++3H2O
��Fe3++3H2O
| ||



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����14�֣�A��B��CΪ3�ֵ��ʣ�����AΪ���壬B��CΪ���壩��D�ı�����Һ�����ˮ�м�����У���Һ�ʺ��ɫ��B��C��Ӧ�IJ���������ˮ�õ���ɫ��ҺE������֮���ת����ϵ����ͼ��

��д���пհף�
��1������A�� ��B�� ��B�����ӽṹʾ��ͼΪ ��
��2��д��������E�ĵ���ʽ�� ��D�ı�����Һ�����ˮ����Һ�ʺ��ɫ��ԭ���ǣ������ӷ���ʽ��ʾ���� ��D��Һ��������ֹѪ�����˵����ֹѪԭ���ǣ� ��
��3����ҵ�ϰ�B������ʯ���鷴Ӧ���Ƶ�Ư�ۣ�Ư�۵���Ч�ɷ��� ��Ư�۳���������ˮ��ɱ��������ԭ���ǣ�д����Ӧ�Ļ�ѧ����ʽ���������ʵ�������
˵������ ��
��4��F�м���NaOH��Һ�����ڿ����з����ɰ�ɫ��Ϊ����ɫ����ɺ��ɫ�����Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��³�����1��02�µ�Ԫ���� ���ͣ������
��6�֣�A��B��CΪ3�ֵ��ʣ�����AΪ���壬B��CΪ���壩��D�ı�����Һ�����ˮ�м�����У���Һ�ʺ��ɫ��B��C��Ӧ�IJ���������ˮ�õ���ɫ��ҺE������֮���ת����ϵ����ͼ��
����д���пհף�
��1������A�� ��B�� ��C�� ��
��2��F�м���NaOH��Һ�����ڿ����з��õĻ�ѧ����ʽ�� �� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��㶫ʡ��ɽ����������ѧ������ѧ��������ѧ�� ���ͣ�ʵ����
����14�֣�A��B��CΪ3�ֵ��ʣ�����AΪ���壬B��CΪ���壩��D�ı�����Һ�����ˮ�м�����У���Һ�ʺ��ɫ��B��C��Ӧ�IJ���������ˮ�õ���ɫ��ҺE������֮���ת����ϵ����ͼ��
��д���пհף�
��1����3�֣�����A�� ��B�� ��B�����ӽṹʾ��ͼΪ ��
��2����5�֣�д��������E�ĵ���ʽ�� ��D�ı�����Һ�����ˮ����Һ�ʺ��ɫ��ԭ���ǣ������ӷ���ʽ��ʾ���� ��D��Һ��������ֹѪ�����˵����ֹѪԭ���ǣ� ��
��3����4�֣���ҵ�ϰ�B������ʯ���鷴Ӧ���Ƶ�Ư�ۣ�Ư�۵���Ч�ɷ��� ��Ư�۳���������ˮ��ɱ��������ԭ���ǣ�д����Ӧ�Ļ�ѧ����ʽ���������ʵ�������
˵������ ��
��4����2�֣�F�м���NaOH��Һ�����ڿ����з����ɰ�ɫ��Ϊ����ɫ����ɺ��ɫ�����Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ��㶫ʡ��ɽ�и�����ѧ��������ѧ�� ���ͣ�ʵ����
����14�֣�A��B��CΪ3�ֵ��ʣ�����AΪ���壬B��CΪ���壩��D�ı�����Һ�����ˮ�м�����У���Һ�ʺ��ɫ��B��C��Ӧ�IJ���������ˮ�õ���ɫ��ҺE������֮���ת����ϵ����ͼ��

��д���пհף�
��1����3�֣�����A�� ��B�� ��B�����ӽṹʾ��ͼΪ ��
��2����5�֣�д��������E�ĵ���ʽ�� ��D�ı�����Һ�����ˮ����Һ�ʺ��ɫ��ԭ���ǣ������ӷ���ʽ��ʾ���� ��D��Һ��������ֹѪ�����˵����ֹѪԭ���ǣ� ��
��3����4�֣���ҵ�ϰ�B������ʯ���鷴Ӧ���Ƶ�Ư�ۣ�Ư�۵���Ч�ɷ��� ��Ư�۳���������ˮ��ɱ��������ԭ���ǣ�д����Ӧ�Ļ�ѧ����ʽ���������ʵ�������
˵������ ��
��4����2�֣�F�м���NaOH��Һ�����ڿ����з����ɰ�ɫ��Ϊ����ɫ����ɺ��ɫ�����Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com