
ij��ɫ��Һ��ֻ��������NH4����K����Al3����Mg2����HCO3����Cl����I����MnO4����SO42���������еļ������ӡ���������ɣ��ֽ�������ʵ�飺
��ȡl0mL����Һ���Թ��еμ�������Ba(NO3��2��Һ����ϡ�����ữ����˵õ�0.03mol��ɫ�����ס�
��ȡ������Ӧ�����Һ������AgNO3��Һδ������������
����ȡl0mL����Һ���Թ��У��μ�NaOH��Һ������ɫ�����ң�������NaOH�����ʵ���Ϊ0. 03 molʱ�����������ﵽ������μ�NaOH��Һ�����ȣ���ʼ������������ռ���������������ɱ����Ϊ0. 224L(�����ȫ���ݳ�������������ȫ�ܽ⡣�����ƶ���ȷ����
A���϶���K+��Al3+��Mg2+��SO42-
B���϶���K+��NH4+��Al3+��SO42��
C���϶�û��K+��HCO3-��MnO4-
D���϶�û��K+��NH4+��Cl-
B
��������
�����������Һ����ɫ�ģ���һ��������MnO4�������ݢٿ�֪��ɫ���������ᱵ�����ԭ��Һ�к���0.03molSO42�������ݢڿ�֪ԭ��Һ�в�����Cl����I������ȡl0mL����Һ���Թ��У��μ�NaOH��Һ������ɫ�����ң�������NaOH�����ʵ���Ϊ0. 03 molʱ�����������ﵽ������μ�NaOH��Һ�����ȣ���ʼ������������ռ���������������ɱ����Ϊ0. 224L(�����ȫ���ݳ�������������ȫ�ܽ⣬��˵����ɫ����һ��������������������0.01mol�����ӣ���˲�����Mg2����HCO3���������ǰ��������ʵ�����0.01mol��˵��ԭ��Һ�к���0.01mol NH4����������Һ�ĵ����Կ�֪0.03mol��2��0.01mol��3+0.01mol��������Һ��һ�������м����ӣ���˴�ѡB��
���㣺�������ӹ����Լ����Ӽ�����й��ж�


 �����Ծ���Ԫ���Ծ�ϵ�д�
�����Ծ���Ԫ���Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �� I��ij��ɫϡ��ҺX�У����ܺ����±����������е�ij���֣�
�� I��ij��ɫϡ��ҺX�У����ܺ����±����������е�ij���֣�| ������ | CO32-��SiO32-��AlO2-��Cl- |
| ������ | Al3+��Cu2+��Mg2+��NH4+��Na+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���϶���Na+��Mg2+��Al3+��Cl- | B���϶���Al3+��SO42-��NH4+ | C���϶�û��K+��HCO3-��MnO4- | D���϶�û��K+��NH4+��Cl- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ������ʡ������ѧ�ڵڶ����¿����ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
��1����Ϊ�ڢ�A��Ԫ�أ����ĵ��ʺͻ�������ijЩ���ʵĻ�ѧ��������������֮������֪��Ԫ�ؾ����������ʣ�
 ��
��




 ��
��
�Իش�
�����������ᣬ����Ӧ�����Һ��ͨ���������йط�Ӧ������������Ӧ�仯����д���йط�Ӧ�Ļ�ѧ����ʽ��_____________________________________________________��_______________________________________��
�ڽ�������Һ���ɺ�����������ù��壬�仯����������FeCl3��Һ��Ӧ�ı仯�������õ��Ĺ���������__________���ѧʽ����
��������SnCl2��Һ������ļ���Һ��Ӧ�ķ�����Sn��OH��2���ü��ѡ��________��
��2��ij��ɫϡ��ҺX�У����ܺ����±����������е�ij���֡�
|
������ |
|
|
������ |
|
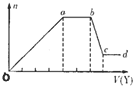
��ȡ����Һ�����������м���ij�Լ�Y���������������ʵ�����n��������Լ�Y�����V���Ĺ�ϵ��ͼ��ʾ��

����Y�����ᣬ����Һ�к��еĽ�����������_________��ab�η�����Ӧ�����ӷ���ʽΪ_______________��ͼ��oa�βμӷ�Ӧ�������ӵ����ʵ���֮��Ϊ___________________��
����Y��NaOH��Һ����bc�η�Ӧ�����ӷ���ʽΪ____________________��
�����������ӵ�ˮ�⣬����H����OH����Ӱ�죬����Һ��ֻ�����������ӣ������ǵ����Ӹ�����Ϊ_____________________________________________������������ǰ���������ں���ǰ���ͼ��ں��˳�����У���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��7�֣�ij��ɫ������Һ��ֻ���ܴ����������������е������֣�K+��SO42����Cl����Ca2+��H+��CO32����Fe3+��С��ͬѧȡ������Һ���ν�������ʵ�飺
����������Һ�еμӹ���BaCl2��Һ���а�ɫ�������ɣ����ó����˳����˳�������ȫ����ϡ���ᡣ
����ٵ���Һ�м���AgNO3��Һ���а�ɫ�������ɣ��ó���������ϡ���ᡣ
��������ʵ�飬�����жϸ���Һ��һ������ �������� ��һ�������� ���������� �����ܺ��� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com