
вдЪЏФЋзіЕчМЋЕчНт200 mLЁЁ0.5 mol/LЕФCuSO4ШмвКЃЌдкБъзМзДПіЯТЃЌвѕМЋЩЯЪеМЏЕН2.24 LЦјЬхЪБЃЌЭЃжЙЭЈЕчЃЎЧыЛиД№ЯТСаЮЪЬтЃК
(1)ећИіЕчНтЙ§ГЬжавѕМЋЕФЕчМЋЗДгІЪНЮЊ________ЃЛ
(2)ЕчНтЙ§ГЬжаЙВЪеМЏЕНЦјЬхЕФЬхЛ§ЮЊ________ЃЛ
(3)ШєЕчНтЧАКѓШмвКЕФЬхЛ§БфЛЏКіТдВЛМЦЃЌЕчНтКѓШмвКЛжИДжСЪвЮТЪБЕФpHжЕдМЮЊ________ЃЛ
(4)ЕчНтНсЪјКѓЃЌгћЪЙШмвКЛжИДЕНЕчНтЧАШмвКЕФзщГЩКЭХЈЖШЃЌПЩМгШы________mol________(ЬюЛЏбЇЪН)ЃЎ

| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКдФЖСРэНт
| ||
| ||
| ЪЕбщзщ | ЮТЖШ/Ёц | Ц№ЪМСП/mol | ЦНКтСП/mol | ДяЕНЦНКтЫљ ашЪБМф/min | ||
| H2O | CO | CO2 | CO | |||
| 1 | 650 | 2 | 4 | 1.6 | 2.4 | 5 |
| 2 | 900 | 1 | 2 | 0.4 | 1.6 | 3 |
| 3 | 900 | a | b | c | d | t |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃККгФЯЪЁЧпбєвЛжа2010НьИпШ§ЕкЫФДЮдТПМЁЂЛЏбЇЪдОэ ЬтаЭЃК022
вдЪЏФЋзіЕчМЋЕчНт200 mLЁЁ0.5 mol/LЕФCuSO4ШмвКЃЌдкБъзМзДПіЯТЃЌвѕМЋЩЯЪеМЏЕН2.24 LЦјЬхЪБЃЌЭЃжЙЭЈЕчЃЎЧыЛиД№ЯТСаЮЪЬтЃК
(1)ЕчНтЙ§ГЬжавѕМЋЕФЕчМЋЗДгІЪНЮЊ________ЁЂ________ЃЛ
(2)ЕчНтЙ§ГЬжаЙВЪеМЏЕНЦјЬхЕФЬхЛ§ЮЊ________ЃЛ
(3)ШєЕчНтЧАКѓШмвКЕФЬхЛ§БфЛЏКіТдВЛМЦЃЌЕчНтКѓШмвКЕФpHжЕдМЮЊ________ЃЛ
(4)ЕчНтНсЪјКѓЃЌгћЪЙШмвКЛжИДЕНЕчНтЧАШмвКЕФзщГЩКЭХЈЖШЃЌПЩМгШы________mol________ЃЛ
(5)ЕчНтЪБЕФЕчСїЧПЖШЮЊ20АВХрЃЌРэТлЩЯЫљашЕчНтЪБМфЮЊ________ЗжЃЎ(Щш1 molЕчзгЕФЕчСПЮЊ96500ПтТи)
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃКЩНЖЋЪЁЪЄРћгЭЬявЛжа2012НьИпШ§ЯТбЇЦкЕквЛДЮЕїбаПМЪдЛЏбЇЪдЬт ЬтаЭЃК022
ЙЄвЕЩЯгУCH4КЭH2OЮЊдСЯЭЈЙ§ЯТСаЗДгІдквЛЖЈЬѕМўЯТжЦБИаТаЭШМСЯМзДМЃЌЗДгІШчЯТЃК
ЂйCH4(g)ЃЋH2O(g)![]() CO(g)ЃЋ3H2(g)ЁЁІЄH1
CO(g)ЃЋ3H2(g)ЁЁІЄH1
ЂкCO(g)ЃЋ2H2(g)![]() CH3OH(g)ЁЁІЄH2
CH3OH(g)ЁЁІЄH2
ЧыАДвЊЧѓЛиД№ЯТСаЮЪЬтЃК
(1)НЋ0.20 molЁЁCH4КЭ0.30 molЁЁH2O(g)ЭЈШыШнЛ§ЮЊ2 LЕФУмБеШнЦїжаЃЌдквЛЖЈЬѕМўЯТЗЂЩњЗДгІЂйЃЌДяЕНЦНКтЪБCH4ЕФзЊЛЏТЪгыЮТЖШЁЂбЙЧПЕФЙиЯЕШчЯТЭМЃЎЕБЮТЖШВЛБфЫѕаЁЬхЛ§ЪБДЫЗДгІЕФФцЗДгІЫйТЪ(ЬюЁАдіДѓЁБЁЂЁАМѕаЁЁБЛђЁАВЛБфЁБ)________ЃЛІЄH1________0ЃЌp1________p2(ОљЬюЁАЃМЁБЁЂЁАЃНЁБЛђЁАЃОЁБ)ЃЛp1ЁЂ100ЁцЪБЦНКтГЃЪ§ЕФжЕKЃН________ЃЎ

(2)ЮЊСЫЬНОПЗДгІЂкКЯГЩМзДМЪЪвЫЕФЮТЖШКЭбЙЧПЃЌФГЭЌбЇЩшМЦСЫвдЯТШ§зщЪЕбщЃЌВПЗжЪЕбщЬѕМўвбОЬюдкЯТУцЪЕбщЩшМЦБэжаЃЌЧыдкПеИёжаЬюШыЪЃгрЕФЪЕбщЬѕМўЪ§ОнЃЎ

Шєдк300ЁцЪБЦфЫћЬѕМўВЛБфЃЌНЋИУЗДгІШнЦїШнЛ§бЙЫѕЕНдРДЕФ1/2ЃЌжиаТЦНКтЪБCH3OHЕФЮяжЪЕФСПЕФБфЛЏЪЧ________ЃЌKЕФБфЛЏЪЧ________(ОљЬюЁАдіДѓЁБЃЌЁАМѕаЁЁБЛђЁАВЛБфЁБ)ЃЎ
(3)вбжЊдкГЃЮТГЃбЙЯТЃК2CH3OH(I)ЃЋ3O2(g)![]() 2CO2(g)ЁЁІЄHЃНЃ1275.6 kJ/mol
2CO2(g)ЁЁІЄHЃНЃ1275.6 kJ/mol
H2O(g)ЃНH2O(I)ЁЁІЄHЃНЃ44.0 kJ/molЃЌЧыМЦЫу32 gаТаЭШМСЯМзДМЭъШЋШМЩеЩњГЩвКЬЌЫЎЗХГіШШСПЮЊ________kJЃЎ
(4)аТаЭШМСЯМзДМЛЙПЩвдгУзіШМЩеЕчГиЃЌЦфЗДгІдРэШчЯТЭМЫљЪОЃЌЕчГиЙЄзїЪБЕчзгвЦЖЏЗНЯђЪЧ(МЋАхгУMЁЂNБэЪО)________ЃЌИКМЋЕчМЋЗДгІЪНЮЊ________ЃЎ

ШєвдИУШМСЯЕчГизїЮЊЕчдДЃЌгУЪЏФЋзїЕчМЋЕчНт500 mLБЅКЭЪГбЮЫЎЃЌЕБСНМЋЙВЪеМЏЕН1.12 L(БъзМзДПіЯТЁЂВЛПМТЧЦјЬхЕФШмНтМАЗДгІ)ЪБЃЌГЃЮТЯТЫљЕУШмвКЕФpHЃН________(МйЩшЗДгІЧАКѓШмвКЬхЛ§ВЛБф)ЃЎ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2013-2014бЇФъЩНЖЋЪЁБѕжнЪаИпШ§3дТФЃФтЃЈвЛФЃЃЉПМЪдЛЏбЇЪдОэЃЈНтЮіАцЃЉ ЬтаЭЃКЬюПеЬт
бЧТШЫсФЦ(NaClO2)ЪЧвЛжжИпаЇбѕЛЏМСЁЂЦЏАзМСЁЃвбжЊЃКNaClO2БЅКЭШмвКдкЮТЖШЕЭгк38oCЪБЮіГіЕФОЇЬхЪЧNaClO2ЁЄ3H2OЃЌИпгк38oCЪБЮіГіОЇЬхЪЧNaClO2ЃЌИпгк60oCЪБNaClO2ЗжНтГЩNaClO3КЭNaClЁЃРћгУЯТЭМЫљЪОзАжУжЦБИбЧТШЫсФЦЁЃзАжУЂкЗДгІШнЦїЮЊШ§ОБЩеЦПЁЃ
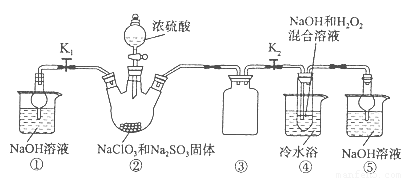
ЭъГЩЯТСаЬюПеЃК
ЃЈ1ЃЉзщзАКУвЧЦїКѓЃЌМьВщећЬззАжУЦјУмадЕФВйзїЪЧЃК??????????????????? ЁЃ
ЃЈ2ЃЉзАжУЂкжаВњЩњClO2ЕФЛЏбЇЗНГЬЪНЮЊ ??????????????????????????????? ЁЃ
зАжУЂмжаЗЂЩњЗДгІЕФРызгЗНГЬЪНЮЊ????????????????????????????????? ЁЃ
ЃЈ3ЃЉДгзАжУЂмЗДгІКѓЕФШмвКЛёЕУNaClO2ОЇЬхЕФВйзїВНжшЮЊЃК
ЂйМѕбЙЃЌ55oCеєЗЂНсОЇЃЛЂк????? ЃЛЂл????? ЃЛЂмЕЭгк60oCИЩдяЃЌЕУЕНГЩЦЗЁЃ
ЃЈ4ЃЉФПЧАвбПЊЗЂГігУЕчНтЗЈжЦШЁClO2ЕФаТЙЄвеЁЃ
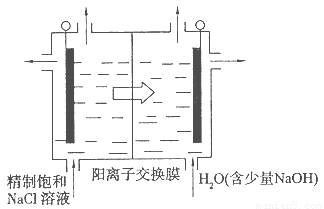
ЂйЩЯЭМЪЧгУЪЏФЋзїЕчМЋЃЌвЛЖЈЬѕМўЯТЕчНтБЅКЭЪГбЮЫЎжЦШЁClO2ЕФЪОвтЭМЁЃдђбєМЋВњЩњC1O2ЕФЕчМЋЗДгІЮЊ ????????????? ЁЃ
ЂкЕчНтвЛЖЮЪБМфЃЌЕБвѕМЋВњЩњЕФЦјЬхЬхЛ§ЮЊ112 mL(БъзМзДПі)ЪБЃЌЭЃжЙЕчНтЁЃЭЈЙ§бєРызгНЛЛЛФЄЕФбєРызгЕФЮяжЪЕФСПЮЊ??? molЁЃ
ЃЈ5ЃЉзМШЗГЦШЁЫљЕУбЧТШЫсФЦбљЦЗl0 gгкЩеБжаЃЌМгШыЪЪСПеєСѓЫЎКЭЙ§СПЕФЕтЛЏМиОЇЬхЃЌдйЕЮШыЪЪСПЕФЯЁСђЫсЃЌГфЗжЗДгІ(ClO2-+4I-+4H+=2H2O+2I2+C1-)ЁЃНЋЫљЕУЛьКЯвКХфГЩ250 mLД§ВтШмвКЁЃХфжЦД§ВтвКашгУЕНЕФЖЈСПВЃСЇвЧЦїЪЧ????? ЃЛШЁ25ЃЎ00 mLД§ВтвКЃЌгУ2ЃЎ0molЁЄL-1Na2S2O3БъзМвКЕЮЖЈ(I2+2S2O32-=2I-+S4O62-)ЃЌвдЕэЗлШмвКзіжИЪОМСЃЌДяЕНЕЮЖЈжеЕуЪБЕФЯжЯѓЮЊ?????????? ЁЃжиИДЕЮЖЈ2ДЮЃЌВтЕУNa2S2O3ШмвКЦНОљжЕЮЊ20ЃЎ00 mLЁЃИУбљЦЗжаNaClO2ЕФжЪСПЗжЪ§ЮЊ???????? ЁЃ
ВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com