
��֪��N2(g)��3H2(g) 2NH3(g)����H����Q kJ��mol��1��Q��0�������м���������ͬ���ܱ���������������г���1mol N2(g)��3mol H2(g)����һ�������´ﵽƽ��ʱ�ų�������ΪQ1 kJ������ͬ���������������г���2mol NH3(g)���ﵽƽ��ʱ���յ�����ΪQ2 kJ����֪Q2��3Q1��������������ȷ����: (�� ��)
2NH3(g)����H����Q kJ��mol��1��Q��0�������м���������ͬ���ܱ���������������г���1mol N2(g)��3mol H2(g)����һ�������´ﵽƽ��ʱ�ų�������ΪQ1 kJ������ͬ���������������г���2mol NH3(g)���ﵽƽ��ʱ���յ�����ΪQ2 kJ����֪Q2��3Q1��������������ȷ����: (�� ��)
A��Q1��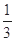 Q Q |
| B���ﵽƽ��ʱ����������H2��ת����Ϊ25�� |
C��ƽ��ʱ�������������ѹǿΪ��ʼʱѹǿ��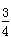 |
| D��ƽ��ʱ��������NH3(g)������������������е�С |
B
�����������������1molN2��3molH2��ȫ��Ӧ��������2molNH3�����Լ���������ϵ��ƽ��״̬��һ���ģ������������Ũ����ȫ��ͬ��N2��H2��ȫ��Ӧʱ����Q����NH3��ȫ�ֽ�ʱ����Ҳ��Q������������е�ת����Ϊx�������Q��x��Q1�������������е�ת����Ϊy����Q��y��Q2������ΪQ2=3Q1������Q��y��3Q��x����y��3x������������ϵ��ƽ��״̬��һ���ģ�����
N2��g��+3H2��g�� 2NH3��g��
2NH3��g��
����ʼ����mol�� 1 3 0
��ת������mol�� x 3x 2x
��ƽ������mol�� 1��x 3��3x 2x
N2��g��+3H2��g�� 2NH3��g��
2NH3��g��
����ʼ����mol�� 0 0 2
��ת������mol�� y 3y 2y
��ƽ������mol�� y 3y 2��2y
����N2����1��x��y
��x��y��1����������������Ҳһ����
��y��3x
����x��25%
��ƽ��ʱ����������ʵ���Ϊ1��0.25��3��0.75��0.5��3.5
����ѹǿ֮�ȵ������ʵ���֮�ȿ�֪
ƽ��ʱ�������������ѹǿΪ��ʼʱѹǿ��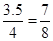
����B��ȷ��C����ȷ���������Ϸ�����֪��Q��x��Q1����Q1��0.25Q������A����ȷ������1molN2��3molH2��ȫ��Ӧ��������2molNH3�����Լ���������ϵ��ƽ��״̬��һ���ģ�ƽ��ʱ��������NH3��g��������������������е���ȣ�����ѡ��D����ȷ����ѡB��
���㣺���黯ѧƽ��ļ���
�����������Ǹ߿��еij������ͣ���Ŀ�Ѷ����У��ۺ���ǿ������������ѧ���������⡢�������������Լ��淶������������������ʱ��Ҫע���Чƽ�����������á�


 ȫ�Ų��Ծ�ϵ�д�
ȫ�Ų��Ծ�ϵ�д� ���100��1�ž�ϵ�д�
���100��1�ž�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)�����ۡ��������ʱ�����Կ���һ��������в�ð������ɫ�����壬����Ϊ�˱�֤���䰲ȫ�ɱ��ջ����Զ��������ų��IJ��ָ�ѹ�������仯�����ģ��ݳ��ĺ���ɫ������_________������������Ļ�ѧ��Ӧ����ʽΪ__________________��
(2)����1 molƫ������ȼ��ʱ��ת�Ƶ��ӵ����ʵ���Ϊ_________��
(3)�е����ػ������(N2H4)��ȼ�ϺͶ���������Ӧ����֪��
N2(g)+2O2(g)====2NO2(g);��H=+67.7 kJ��mol-1
N2H4(g)+O2(g)====N2(g)+2H2O(g);��H=-534 kJ��mol-1
���й����º�NO2��Ӧ���Ȼ�ѧ����ʽ����ȷ����( )
A.2N2H4(g)+2NO2(g)====3N2(g)+4H2O(l);��H=-1 135.7 kJ��mol-1
B.2N2H4(g)+2NO2(g)====3N2(g)+4H2O(g);��H=-1 000.3 kJ��mol-1
C.N2H4(g)+NO2(g)====3/2N2(g)+2H2O(l):��H=-1 135.7 kJ��mol-1
D.2N2H4(g)+2NO2(g)====3N2(g)+4H2O(g);��H=-1 135.7 kJ��mol-1
�����Ӧ���ڻ���ƽ��������ͷŴ������ȺͿ��ٲ������������⣬����һ���ܴ���ŵ���_____________________________________________________________________��
(4)ͨ���������������������Ͷ�������������ת����N2O4(g)(��ɫ)![]() 2NO2(g)(����ɫ)����H��0����֪���������н�ǿ�������ԣ�������˵���������( )
2NO2(g)(����ɫ)����H��0����֪���������н�ǿ�������ԣ�������˵���������( )
A.N2O4��NO2��Ϊͬ��������
B.��̬ʱ��1 mol N2O4����ȫ�ֽ��2 mol NO2
C.����NO2(g)����������ѡ�õ��ۡ��⻯����Һ
D.N2O4��NO2�������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
4NH3(g)+5O2(g)![]() 4NO(g)+6H2O(g)����H=-905 kJ��mol-1
4NO(g)+6H2O(g)����H=-905 kJ��mol-1
2H2(g)+O2(g)![]() 2H2O����H=-483.6 kJ��mol-1
2H2O����H=-483.6 kJ��mol-1
��N2(g)+3H2(g)![]() 2NH3(g)�Ħ�H=________��
2NH3(g)�Ħ�H=________��
(2)��ҵ����һ���¶��£���һ������N2��H2ͨ�뵽���Ϊ
������ѹǿ������Ӧ���Ũ�Ȣ�ʹ�ô����ܽ����¶�
(3)����ѧ��ӦN2(g)+2H2(g) ![]() 2NH3(g)�ﵽƽ���ı�ijЩ����(���ı�N2��H2��NH3������)����Ӧ�����뷴Ӧʱ��Ĺ�ϵ����ͼ�����б�ʾƽ��������NH3�ĺ�����ߵ�һ��ʱ����___________________�����¶�ΪT ��ʱ����
2NH3(g)�ﵽƽ���ı�ijЩ����(���ı�N2��H2��NH3������)����Ӧ�����뷴Ӧʱ��Ĺ�ϵ����ͼ�����б�ʾƽ��������NH3�ĺ�����ߵ�һ��ʱ����___________________�����¶�ΪT ��ʱ����

(4)�ϳɰ���ԭ��������һ�����͵���ɫ��Դ�����й����ķ�չǰ������������ȼ�ϵ�ؽ�����ͼ��ʾʵ�飺

����д������ȼ�ϵ���еĵ缫��Ӧʽ��
������____________________________________________________________��
������____________________________________________________________��
����ͼװ���У�ijһͭ�缫������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(16��) ��ԴΣ���ǵ�ǰȫ�����⣬��Դ������Ӧ����ԴΣ������Ҫ�ٴ롣
������������������Դ����Դ���������� �� ������ĸ����
a��������չũ���������������Ľո�ת��Ϊ����Ч����Դ
b����������ú��ʯ�ͺ���Ȼ������������������������Դ����
c������̫���ܡ�ˮ�ܡ����ܡ����ȵ�����Դ������ʹ��ú��ʯ�͵Ȼ�ʯȼ��
d��������Դ���ģ�������Դ���ظ�ʹ�á���Դ��ѭ������
(2)���ʯ��ʯī��Ϊ̼��ͬ�������壬����ȼ����������ʱ����һ����̼�����ȼ�����ɶ�����̼����Ӧ�зų�����������ͼ��ʾ��
��a����ͨ��״���£����ʯ��ʯī��____��___������ʯ����ʯī�������ȶ���ʯī��ȼ����Ϊ____��___ kJ��mol��1��
��b��12 gʯī��һ����������ȼ�գ���������36g���ù��̷ų������� �� kJ��
(3)��֪��N2(g)��O2(g)��2NO(g)����H��+180.0 kJ��mol��1��
�ۺ������й���Ϣ����д��CO��NO���Ȼ�ѧ����ʽ �� ��
����˹̹����ѧ�о���Ա�������һ�֡�ˮ����أ����ֵ�������õ�ˮ�뺣ˮ֮�京�����IJ����з��硣
��4���о���������ص������ö����������װ�Ϊ���Ͽ���߷���Ч�ʣ������������ײ��Ͼ���
�� ���ԣ����������ӳ�ֽӴ���
��5����ˮ�еġ�ˮ������ܷ�Ӧ�ɱ�ʾΪ��5MnO2��2Ag��2NaCl��Na2Mn5O10��2AgCl���õ�ظ�����ӦʽΪ �� ��������1 mol Na2Mn5O10ת�� �� mol���ӡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011������ʡ���Ը���ѧ����1���¿������ۣ���ѧ���� ���ͣ������
��1����֪��N2(g)+O2(g)=2NO(g)����H= +180.5kJ/mol
4NH3(g)+5O2(g)=4NO(g)+6H2O(g)����H=��905kJ/mol
2H2(g)+O2(g)=2H2O(g)����H=��483.6kJ/mol
��N2(g)+3H2(g)=2NH3(g)�ġ�H= ��
��2����ҵ�ϳɰ��ķ�ӦΪN2(g)+3H2(g)  2NH3(g)����һ���¶��£���һ������N2��H2ͨ��̶����Ϊ1L���ܱ������дﵽƽ��ı�������������ʹƽ��������Ӧ�����ƶ����� ��
2NH3(g)����һ���¶��£���һ������N2��H2ͨ��̶����Ϊ1L���ܱ������дﵽƽ��ı�������������ʹƽ��������Ӧ�����ƶ����� ��
������ѹǿ ��ͨ��He
��ʹ�ô��� �ܽ����¶�
��3����ҵ�ϳɰ��ķ�ӦΪN2(g)+3H2(g)  2NH3(g)�������ݻ�Ϊ2.0L���ܱ������г���0.60molN2(g)��1.60molH2(g)����Ӧ��һ�������´ﵽƽ��ʱ��NH3�����ʵ���������NH3�����ʵ����뷴Ӧ��ϵ���ܵ����ʵ���֮�ȣ�Ϊ
2NH3(g)�������ݻ�Ϊ2.0L���ܱ������г���0.60molN2(g)��1.60molH2(g)����Ӧ��һ�������´ﵽƽ��ʱ��NH3�����ʵ���������NH3�����ʵ����뷴Ӧ��ϵ���ܵ����ʵ���֮�ȣ�Ϊ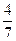 ������������´ﵽƽ��ʱN2ת����Ϊ ��
������������´ﵽƽ��ʱN2ת����Ϊ ��
��4���ϳɰ���ԭ��������һ�����͵���ɫ��Դ�����й����ķ�չǰ������������ȼ�ϵ�ؽ�����ͼ��ʾʵ�飺������c��d��Ϊ̼����NaCl��Һ�����Ϊ500ml��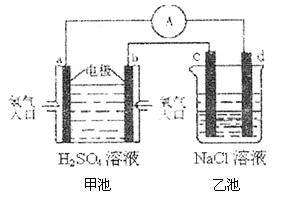
��b��Ϊ �����缫��Ӧʽ ��
c��Ϊ �����缫��Ӧʽ 
����ͼװ���У���b�������ĵ�O2�ڱ�״���µ����Ϊ280mlʱ�����ҳ���Һ��PHΪ �����跴Ӧǰ����Һ������䣬��NaCl��Һ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com