
 2CaSO4+2Cl2��+2H2O�����������������ȡ��������֤�����ʵ�ʵ�飮
2CaSO4+2Cl2��+2H2O�����������������ȡ��������֤�����ʵ�ʵ�飮

 �����͵�b���ʴ�Ϊ��b��
�����͵�b���ʴ�Ϊ��b�� n��Na2S2O3��=20.0 mL×10-3 L?mL-1×0.1 mol?L-1×
n��Na2S2O3��=20.0 mL×10-3 L?mL-1×0.1 mol?L-1× =0.005 mol��
=0.005 mol�� ×100%=35.75%��
×100%=35.75%��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijУ��ѧ�о���ѧϰС����������˽�������ݣ�
�Ҷ��ᣨHOOC-COOH���ɼ�дΪH2C2O4���׳Ʋ��ᣬ������ˮ�����ڶ�Ԫ��ǿ�ᣨΪ������ʣ���������ǿ��̼�ᣬ���۵�Ϊ101.5�棬��157��������Ϊ̽������IJ��ֻ�ѧ���ʣ�����������ʵ�飺
����ʢ��1 mL����NaHCO3��Һ���Թ��м��������Ҷ�����Һ���۲쵽����ɫ���ݲ������÷�Ӧ�����ӷ���ʽΪ________________________________��
����ʢ���Ҷ��ᱥ����Һ���Թ��е��뼸�������ữ��KMnO4��Һ������������Һ���Ϻ�ɫ��ȥ��˵���Ҷ������_____________��������ԡ�������ԭ�ԡ������ԡ���������ƽ�÷�Ӧ�����ӷ���ʽ��
____ MnO4�C + ____ H2C2O4 + _____ H+ = _____ Mn2+ + _____ CO2�� + _____ H2O
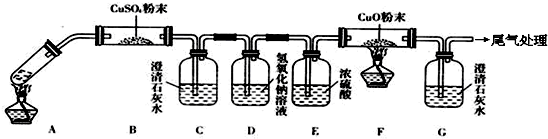
 �ǽ�һ�������Ҷ�������Թ��У�����ͼ��ʾװ�ý���ʵ�飨�г�װ��δ�������
�ǽ�һ�������Ҷ�������Թ��У�����ͼ��ʾװ�ý���ʵ�飨�г�װ��δ�������
ʵ�鷢�֣�װ��C��G�г���ʯ��ˮ����ǣ�B��CuSO4��ĩ������F��CuO��ĩ��졣�ݴ˻ش�
����װ���У�D��������__________________���Ҷ���ֽ�Ļ�ѧ����ʽΪ______________________��
�ȸ�С��ͬѧ��2.52 g���ᾧ�壨H2C2O4��2H2O�����뵽100 mL 0.2 mol��L��1��NaOH��Һ�г�ַ�Ӧ����÷�Ӧ����Һ�����ԣ���ԭ����___________________________________�������ּ�������
��������Һ�и����ӵ�Ũ���ɴ�С��˳��Ϊ��_____________________________�������ӷ��ű�ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
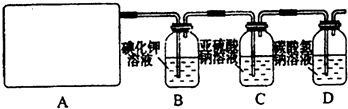
��10�֣�ij�о���ѧϰС��������ϵ�֪��Ư�������ᷴӦ�����Ƶ���������ѧ����ʽΪ��Ca(ClO)2+CaCl2+2H2SO4![]() 2CaSO4+2Cl2��+2H2O�����������������ȡ��������֤�����ʵ�ʵ�顣
2CaSO4+2Cl2��+2H2O�����������������ȡ��������֤�����ʵ�ʵ�顣
�Իش�
��1����ʵ����A���ֵ�װ���� ����дװ�õ���ţ���
��2���������һ��ʵ�飬֤��ϴ��ƿC�е�Na2SO3�Ѿ�������������ʵ�鲽�裩��
��
��3��д��Dװ���з�����Ӧ�����ӷ���ʽ ��
��4����ʵ��������Ե�ȱ�ݣ���������Ľ��ķ��� ��
��5����С���ֽ���������ʵ�飺��ȡƯ��2.0 g ����ĥ���ܽ⣬���Ƴ�250 ml ��Һ��ȡ25 ml ���뵽��ƿ�У��ټ��������KI��Һ������H2SO4��Һ�����á�����ȫ��Ӧ����0.1 mol/L��Na2S2O3��Һ������Һ�ζ���Ӧ���ɵĵ⣬��֪��ӦʽΪ��
2Na2S2O3+ I2 ��Na2S4O6 + 2NaI
��Ӧ���ʱ������ȥNa2S2O320.0 ml�����Ư����Ca(ClO)2����������Ϊ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com