
����Ŀ������������Ԫ��W��X��Y��Z��R��ԭ��������������A��B��C��D��E��F����������Ԫ����ɵ���ѧ��ѧ�������ʣ�����A����Ԫ�����C����ʹʪ���ɫʯ����ֽ���������壬D�ǵ���ɫ���廯���E�ǵ��ʡ�������֮���������ͼת����ϵ(���ֲ���δ���)������˵������ȷ����
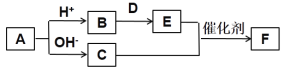
A. �����Ӱ뾶��С��ϵ��Y��Z��R
B. �������ӵĻ�ԭ�ԣ�W��Y��Z
C. �⻯��ķе㣺Y��Z
D. C��E��Ӧ����F�ǹ�ҵ���������Ҫ��Ӧ֮һ
���𰸡�C
��������
����������Ԫ��W��X��Y��Z��R��ԭ��������������A��B��C��D��E��F����������Ԫ����ɵ���ѧ��ѧ�������ʣ�����C����ʹʪ���ɫʯ����ֽ���������壬��CΪNH3��D�ǵ���ɫ���廯�����DΪNa2O2��E�ǵ��ʣ�����C+E��F��֪��ӦΪ���Ĵ���������EΪO2��FΪNO��A����Ԫ�������A�������ᷴӦ����B����Na2O2��Ӧ����O2������Ӧ����NH3����AΪ(NH4)2CO3��NH4HCO3�����WΪHԪ�أ�XΪCԪ�أ�YΪNԪ�أ�ZΪOԪ�أ�RΪNaԪ�أ��ݴ˴��⡣
A.YΪNԪ�أ�ZΪOԪ�أ�RΪNaԪ�أ����Ӳ���Խ�࣬�뾶Խ������ͬ���Ӳ�ṹ�����ӣ�Ԫ�صĺ˵����Խ�����Ӱ뾶ԽС�����Լ����Ӱ뾶��С��ϵ��N3-��O2-��Na+����A��ȷ��
B.WΪHԪ�أ�YΪNԪ�أ�ZΪOԪ�أ�Ԫ�صķǽ�����Խǿ�����Ӧ�������ӵĻ�ԭ��Խ�����ǽ�����O��N��H�����������ӵĻ�ԭ�ԣ�H��N��O����B��ȷ��
C.YΪNԪ�أ�ZΪOԪ�أ�N��O����Ԫ�ص��⻯�ˮ���Ӽ䡢�����Ӽ䶼���������ˮ���Ӽ������Ȱ����Ӽ�����ǿ������ˮ�ķе��Щ�������⻯��ķе㣺Z��Y����C����
D.��ҵ�����ð��Ĵ���������һ����������ȡ�������Ҫ��Ӧ֮һ����D��ȷ��
��ѡC��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���η��ᣨ���������ᣩ�ǽ���ӱ����������Ӧ���Լ�ǿ�����ñ�����ϡ�͵ķ�����ϸ���ϻ���ͨ���Ƶã�F2+H2O�THOF+HF���÷�Ӧ��ˮ�����������з�Ӧ��ˮ��������ͬ����
A. ��������ˮ��Ӧ������ B. ����ˮ��Ӧ������
C. ������ˮ��Ӧ�ƴ����� D. ����������ˮ��Ӧ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ҹ������ƾõ���ʷ������������ʪ����ͭ(Fe+CuSO4=Cu+FeSO4),�Իش��������⡣
��1��Cu2+��δ�ɶԵ�������______����H��O��S�縺���ɴ�С��˳��Ϊ_______��
��2��������ͭ��Һ�еμӹ�����ˮ���γ�[Cu(NH3)4]SO4��ɫ��Һ��[Cu(NH3)4]SO4�л�ѧ��������_______������������ԭ���ӻ�����Ϊ______��
��3����ͭ�Ͻ�������Ϊ_____�����ĵ���(I3)�͵���(I4)�����ֱܷ�Ϊ2957kJ/mol��5290kJ/mol���Ƚ����ݲ�����ԭ��________________��
��4����ͭ�Ͻ��һ�־���ṹΪ�������ͣ���ͼ��ʾ����֪�úϽ���ܶ�Ϊdg/cm3,�����ӵ�����ֵΪNA��������ԭ�Ӽ���С��϶Ϊapm(1pm=10-10cm)����ͭԭ�ӵİ뾶Ϊ_______cm(д���������ʽ)��
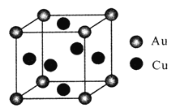
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����л������У������ɵ���ֱ�ӻ��϶��õ�����
A.NH3B.FeSC.FeCl2D.FeCl3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���յ���һ�ţ�sudan ����һ��ż��Ⱦ�ϣ�������ΪʳƷ���Ӽ�ʹ�á������ɱ�����2������Ϊ��Ҫԭ���Ʊ��ģ����ǵĽṹ��ʽ������ʾ��
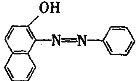
![]()
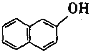
���յ���һ�ţ� �������� ��2-���ӣ�
����ʾ�� �ɱ�ʾΪ
�ɱ�ʾΪ![]() ��
��
��1���յ���һ�ŵĻ�ѧʽ������ʽ��Ϊ______��
��2�������滯����(A)��(D)�У���2�����ӻ�Ϊͬ���칹����У�����ĸ���ţ�_____��




��A�� ��B�� ��C�� ��D��
��3�����������C�����еĹ�������_____��
��4�����ʵ��������£�2�����Ӿ���Ӧ�ɵõ����㻯����E(C8H6O4)��1molE��������̼��������Һ��Ӧ�ɷų�������̼44.8L(��״��)��E�������д�������ʱ��Ӧֻ����������һ��������һ�����Ľṹ��ʽ�ֱ���______________________________��E��̼�����Ʒ�Ӧ�Ļ�ѧ����ʽ��____________________��
��5������E�������Ҵ���Ũ���������¼��ȣ���������һ����ѧʽ������ʽ��ΪC12H14O4���»�����÷�Ӧ�Ļ�ѧ����ʽ��____________________����Ӧ������____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ij�¶�ʱ����һ��10L�ĺ��������У�X��Y��Z��Ϊ���壬�������ʵ����ʵ�����ʱ��ı仯������ͼ��ʾ��������ͼ��������գ�
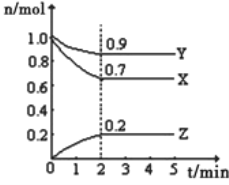
��1��ƽ��ʱ�����ڻ�������ܶȱ���ʼʱ_____�����������������С�������������ͬ������������ƽ����Է�����������ʼʱ_____��
��2����amolX��b mol Y�Ļ�����巢��������Ӧ����Ӧ��ijʱ�̸����ʵ���ǡ�����㣺n��X��=n��Y��=2n��Z������ԭ���������a��b=_____��
���ں��º��ݵ��ܱ������У����������������ٷ����仯ʱ���ٻ�������ѹǿ �ڻ��������ܶ� �ۻ������������ʵ��� �ܻ�������ƽ����Է������� �ݻ���������ɫ ����Ӧ���������ķ�Ӧ����֮�ȵ��ڻ�ѧ������֮�ȡ�
��1��һ����֤��2SO2(g)��O2(g)![]() 2SO3(g)�ﵽƽ��״̬����_____������ţ���ͬ����
2SO3(g)�ﵽƽ��״̬����_____������ţ���ͬ����
��2��һ����֤��I2(g)��H2(g)![]() 2HI(g)�ﵽƽ��״̬����______��
2HI(g)�ﵽƽ��״̬����______��
��3��һ����֤��A(s)��2B(g)![]() C(g)��D(g)�ﵽƽ��״̬����_____����ע��B,C,D��Ϊ��ɫ���ʣ�
C(g)��D(g)�ﵽƽ��״̬����_____����ע��B,C,D��Ϊ��ɫ���ʣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ӷ���ʽ��ȷ���ǣ� ��
A. ��������Һ������ȩ�е�ȩ����CH3CHO+2Ag(NH3)2+ +2OH�� ![]() CH3COONH4 +3NH3+2Ag��+H2O
CH3COONH4 +3NH3+2Ag��+H2O
B. ��������Һ��ͨ������CO2��CO2 +H2O+2C6H5O����2C6H5OH+CO32��
C. �������е���AgNO3��Һ����������Ԫ�أ�Cl��+Ag+=AgCl��
D. �������������ͭ����Һ�У�2CH3COOH+Cu(OH)2=Cu2++2CH3COO-+2H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������йص������Һ��˵������ȷ����
A. ��![]() ��Һ��ͨ��
��Һ��ͨ��![]() ��
��![]() ��С
��С
B. ��![]() ��
��![]() ��Һ��
��Һ��![]() ������
������![]() ��
��![]() ����
����
C. ��![]() ��HF��Һ�еμ�NaOH��Һ�����ԣ�
��HF��Һ�еμ�NaOH��Һ�����ԣ�![]() =1
=1
D. ��![]() ��
��![]() ��Һ�м�������ˮ��
��Һ�м�������ˮ��![]() ����
����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com