


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2009?�Ϻ�����������Դ�ı��⣬�̲��ŷḻ�Ļ�ѧԪ�أ����ȡ��塢��ȣ�
��2009?�Ϻ�����������Դ�ı��⣬�̲��ŷḻ�Ļ�ѧԪ�أ����ȡ��塢��ȣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������Դ�ı��⣬�̲��ŷḻ�Ļ�ѧԪ�أ����ȡ��塢��ȡ�
(1)�ڹ��������£�������������Ӧ�������£�
��![]() ��
��![]() ��
��![]() ����
����
��Ӧ�����γɵĻ�����ĵ���ʽΪ ����Ӧ���б��ƻ��Ļ�ѧ������ ��(����ԡ��Ǽ��ԡ�)��
(2)�ڶ���������Ԫ���У���Ԫ�ؼ���������Ԫ�ص�ԭ�Ӱ뾶�Ӵ�С��˳���� (��Ԫ�ط��ű�ʾ)������Ԫ��ͬ�����ҽ�������ǿ��Ԫ��λ�����ڱ��ĵ� ���� �塣
(3)±�ص��ʼ������������������϶������ŵݱ���ɡ������й�˵����ȷ���� ��
a��±��������ɫ��AgCl��AgBr��AgI ��˳�����μ���
b��±����ļ�����H�DF��H�DC1��H�DBr��H�DI��˳�����μ�С
c��±����Ļ�ԭ��HF��HCl��HBr��HI��˳�����μ���
d��±�ص������������ϰ�![]() ��
��![]() ��
��![]() ��
��![]() ��˳�����ѱ���
��˳�����ѱ���
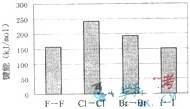
 (4)±�ص��ʵļ��ܴ�С����ͼ����ͼ�ƶϣ�
(4)±�ص��ʵļ��ܴ�С����ͼ����ͼ�ƶϣ�
�ٷǽ�����ǿ��±�أ��䵥�ʷ��ӵĻ�ѧ�� ����(����ס������ס���һ�����ס�)��
��±�ص��ʼ��ܴ�С������Ĺ�ϵΪ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�����ʡ��ˮһ�и�����һ�ָ�ϰ��⻯ѧ�Ծ� ���ͣ������
��8�֣���������Դ�ı��⣬�̲��ŷḻ�Ļ�ѧԪ�أ����ȡ��塢��ȡ�
(1)�ڹ��������£�������������Ӧ�������£���Cl2��Cl+Cl ��Cl+H2��HCl+H ��H+Cl2��HCl+Cl ������Ӧ�����γɵĻ������ ����ʽΪ ����Ӧ���б��ƻ��Ļ�ѧ����
����ʽΪ ����Ӧ���б��ƻ��Ļ�ѧ���� �� ��(����ԡ��Ǽ��ԡ�)��
�� ��(����ԡ��Ǽ��ԡ�)��
(2)�ڶ���������Ԫ���У���Ԫ�ؼ���������Ԫ�ص�ԭ�Ӱ뾶�Ӵ�С��˳���� (��Ԫ�ط��ű�ʾ)������Ԫ��ͬ�����ҽ�������ǿ��Ԫ�� λ�����ڱ��ĵ�
λ�����ڱ��ĵ�  ���� �塣
���� �塣
( 3)±�ص��ʼ���
3)±�ص��ʼ��� ���������������϶������ŵݱ���ɡ������й�˵����ȷ���� ��
���������������϶������ŵݱ���ɡ������й�˵����ȷ���� ��
a��±��������ɫ��AgCl��AgBr��AgI ��˳�����μ���
b��±����ļ�����H��F��H��C1��H��Br��H��I��˳�����μ�С
c��±�� ��Ļ�ԭ��HF��HCl��HBr��HI��˳�����μ���
��Ļ�ԭ��HF��HCl��HBr��HI��˳�����μ���
d��±�ص������������ϰ�F2��Cl2��Br2��I2��˳�����ѱ���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com