
 ���ΪԪ�����ڱ���һ���֣������Ԫ�آ١����ڱ��е�λ�ã��ش��������⣺
���ΪԪ�����ڱ���һ���֣������Ԫ�آ١����ڱ��е�λ�ã��ش��������⣺ ��
������ ��Ԫ�������ڱ��е�λ�ÿ�֪������H������C������N������O������Na������Al������Si������Cl��
��1���ؿ��к�����ߵĽ���Ԫ��Ϊ��Ԫ�أ�
��2�����γɵĵ���Ϊ������������ˮ��Ӧ��������ʹ����
��3��Ԫ�صķǽ�����Խǿ��ԭ�ӵĵõ��ӵ�������Խǿ����۵ĺ���������Ծ�Խǿ����̬�⻯����ȶ���ҲԽǿ��
��4���ɢܢݢ�Ԫ���γɵļȺ������Ӽ��ֺ��й��ۼ�������ΪNaClO�ȣ�
��5����Ԫ�آں͢��γɵĻ������Ӳ�ȱȽ��ʯ��������������γɵľ���Ϊԭ�Ӿ��壬����ԭ�Ӿ��ݿ��ܾ��е����ʻ��ص��жϣ�
��6���ٺ͢��γɵ�ijҺ̬������Ħ��������������ͬ���������ΪN2H4���ڳ��³�ѹ��0.25mol��Һ̬��������������������ȫȼ�����ɵ�����Һ̬ˮ��ͬʱ�ų�QkJ����������1molN2H4��Ӧ�ɷų�4QkJ��������ע�����ʵľۼ�״̬�뷴Ӧ����д�Ȼ�ѧ����ʽ��
��� �⣺��Ԫ�������ڱ��е�λ�ÿ�֪������H������C������N������O������Na������Al������Si������Cl��
��1���ؿ��к�����ߵĽ���Ԫ��Ϊ��Ԫ�أ���Ԫ�������ڱ��е�λ���ǵ�3���ڢ�A�壬
�ʴ�Ϊ����3���ڢ�A�壻
��2�����γɵĵ���Ϊ������������ˮ��Ӧ��������ʹ����ᣬ��Ӧ�����ӷ���ʽΪCl2+H2O?HClO+H++Cl-���ʴ�Ϊ��Cl2+H2O?HClO+H++Cl-��
��3��Ԫ�صķǽ�����Խǿ��ԭ�ӵĵõ��ӵ�������Խǿ����۵ĺ���������Ծ�Խǿ����̬�⻯����ȶ���ҲԽǿ������a��c��ȷ��
�ʴ�Ϊ��a��c��
��4���ɢܢݢ�Ԫ���γɵļȺ������Ӽ��ֺ��й��ۼ�������ΪNaClO�ȣ������ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��5����Ԫ�آں͢��γɵĻ������Ӳ�ȱȽ��ʯ��������������γɵľ���Ϊԭ�Ӿ��壬����ԭ�Ӿ��ݿ�֪���������۵�ܸߡ������ڵ������ӡ����пռ���״�ṹ��ԭ�Ӿ���һ�㲻���磬
��ѡ���٢ۢܣ�
��6���ٺ͢��γɵ�ijҺ̬������Ħ��������������ͬ���������ΪN2H4���ڳ��³�ѹ��0.25mol��Һ̬��������������������ȫȼ�����ɵ�����Һ̬ˮ��ͬʱ�ų�QkJ����������1molN2H4��Ӧ�ɷų�4QkJ���������÷�Ӧ���Ȼ�ѧ����ʽΪ��N2H4��l��+O2��g���TN2��g��+2H2O��l����H=-4Q kJ•mol-1��
�ʴ�Ϊ��N2H4��l��+O2��g���TN2��g��+2H2O��l����H=-4Q kJ•mol-1��
���� ���⿼��λ��Ԫ�����ڱ���Ԫ�������ɣ��Ƕ�ѧ���ۺ������Ŀ��飬��Ŀ�ѶȲ���ע������ԡ��ǽ�����ǿ���Ƚ���ʵ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CO2 | B�� | PCl5 | C�� | NH3 | D�� | HClO |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

 ���÷��ӵĺ˴Ź���������2�ַ壮
���÷��ӵĺ˴Ź���������2�ַ壮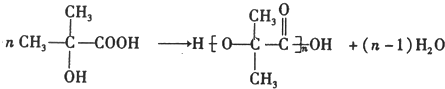 ��F��G�ķ�Ӧ����CH3��2C��OH��-CHO+2Cu��OH��2$\stackrel{��}{��}$��CH3��2C��OH��-COOH+Cu2O��+2H2O��
��F��G�ķ�Ӧ����CH3��2C��OH��-CHO+2Cu��OH��2$\stackrel{��}{��}$��CH3��2C��OH��-COOH+Cu2O��+2H2O���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 ��ͼ��ʾ������ƿ����ˮ����Һ©���ڵ�Һ��Ҳ��ˮ�����ձ��ڵμ�ˮʱ������U�ι���Һ���������ƣ��ָ���ԭ�¶Ⱥ�Һ��������ұ�����ƽ�����ձ��ڵ������ǣ�������
��ͼ��ʾ������ƿ����ˮ����Һ©���ڵ�Һ��Ҳ��ˮ�����ձ��ڵμ�ˮʱ������U�ι���Һ���������ƣ��ָ���ԭ�¶Ⱥ�Һ��������ұ�����ƽ�����ձ��ڵ������ǣ�������| A�� | �������� | B�� | ������ | C�� | �� | D�� | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 | �� | �� | �� | �� | �� | �� | �� | �� |
| ԭ�Ӱ뾶��10-10m�� | 0.74 | 1.60 | 1.52 | 1.10 | 0.99 | 1.86 | 0.75 | 0.82 |
| �����ͻ��ϼ� | +2 | +1 | +5 | +7 | +1 | +5 | +3 | |
| -2 | -3 | -1 | -3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 ��ͼ��ʾ���Թܢ���ʢ��98���ˮ���Թܢ��г�������B����Һ��A�����Թܢ��У���ַ�Ӧ�����K�������Թܢ��е�ˮ���̷��ڣ���A��B�����ǣ�������
��ͼ��ʾ���Թܢ���ʢ��98���ˮ���Թܢ��г�������B����Һ��A�����Թܢ��У���ַ�Ӧ�����K�������Թܢ��е�ˮ���̷��ڣ���A��B�����ǣ�������| A�� | �������� | B�� | ϡ������һ����̼ | ||
| C�� | ����ʳ��ˮ������ | D�� | Ũ��ˮ����ϩ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | $\frac{28m}{n}$ | B�� | $\frac{14m}{n}$ | C�� | $\frac{n}{28m}$ | D�� | $\frac{n}{14m}$ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢� | B�� | �ܢ� | C�� | �ڢ� | D�� | �ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
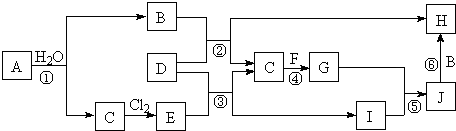
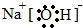 ��
���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com