
��Al2(SO4)3��AlCl3�Ļ����Һ����μ���1 mol��L��1 Ba(OH)2��Һ������������Ba(OH)2��Һ����������ó��������ʵ����Ĺ�ϵ��ͼ������˵������ȷ���� (����)��

A��ͼ��C����Ԫ�ش�����ʽ��AlO
B����D����Һ��ͨ��CO2���壬����������ɫ����
C��ԭ�����Һ��c[Al2(SO4)3]��c(AlCl3)��1��2
D��OA�η�Ӧ�����ӷ���ʽΪ2Al3����3SO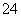 ��3Ba2����8OH��===2AlO
��3Ba2����8OH��===2AlO ��3BaSO4����4H2O
��3BaSO4����4H2O
������C��ΪBaSO4�����������ʵ���Ϊ3 mol����ʱ��Ԫ����AlO ����ʽ���ڣ�B��ΪAl(OH)3��BaSO4��������ʱAl(OH)3�����ʵ���Ϊ7 mol��3 mol��4 mol����ԭ���Һ����1 mol Al2(SO4)3��AlCl3Ϊ4 mol��1 mol��2��2 mol����c[Al2(SO4)3]��c(AlCl3)��1��2��OA�η�Ӧ�����ӷ���ʽΪ2Al3����3SO
����ʽ���ڣ�B��ΪAl(OH)3��BaSO4��������ʱAl(OH)3�����ʵ���Ϊ7 mol��3 mol��4 mol����ԭ���Һ����1 mol Al2(SO4)3��AlCl3Ϊ4 mol��1 mol��2��2 mol����c[Al2(SO4)3]��c(AlCl3)��1��2��OA�η�Ӧ�����ӷ���ʽΪ2Al3����3SO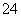 ��3Ba2����6OH��===3BaSO4����2Al(OH)3����
��3Ba2����6OH��===3BaSO4����2Al(OH)3����
�𰸡�D


 �ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
�ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊ������ˮ��c(HClO)��Ӧ��ȡ�Ĵ�ʩ�� (����)��
A������NaOH���塡 B������ʳ�ι���
C������CaCO3�� D������Na2SO3����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
CuSO4��һ����Ҫ�Ļ���ԭ�ϣ����й��Ʊ�;������������ͼ��ʾ������˵����ȷ���� (����)��

A�������;���ڣ�;���ٸ��õ���������ɫ��ѧ˼��
B��Y��������������Һ
C��X������SO2��SO3�Ļ������
D����CuSO4��Һ�����������������ɣ����Ƶõ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������ͭ�Ƚ������仯�������ճ�������Ӧ�ù㷺�����������ʵ��ش����⡣
(1)�����к���һ����̼������X(Fe3C)��X�������Ŀ����и������գ������д��ԵĹ���Y����Y���ڹ����������Һ�д������ڵ���������______________��Y�����Ũ���ᷴӦ����Һ�к��е��εĻ�ѧʽΪ______________________________________________________________��
(2)ij��Һ����Mg2����Fe2����Al3����Cu2�������ӣ������м��������NaOH��Һ���ˣ��������������գ��������պ�Ĺ���Ͷ�������ϡ�����У�������Һ��ԭ��Һ��ȣ���Һ�д������ٵ���������________��
A��Mg2���� B��Fe2����
C��Al3���� D��Cu2��
(3)����������Ҫ�Ĺ�ҵ���ϣ��÷���м�Ʊ������������£�
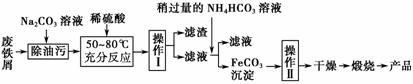
�ش��������⣺
�ٲ������������________���������������________��������ķ���Ϊ______________________________________________________________��
����д������FeCO3���������ӷ���ʽ��_________________________ _______________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ʵ���Ϊ0.10 mol��þ����ֻ����CO2��O2��������������ȼ��(���ﲻ��̼��þ)����Ӧ�������ڹ������ʵ�����������Ϊ (����)��
A��3.2 g�� B��4.0 g��
C��4.2 g�� D��4.6 g
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ж�̼�����Ʒ�ĩ�л���̼���Ƶ�ʵ�鷽���� (����)��
A������ʱ������ų�
B���μ�����ʱ�����ݷų�
C������ˮ��μ�ϡBaCl2��Һ�а�ɫ��������
D������ˮ��μӳ���ʯ��ˮ�а�ɫ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ʵ������Ͳ���ȷ���� (����)��
A�����з�̪��NaHCO3��Һ��dz��ɫ���Ⱥ��ɫ�������ΪNaHCO3�ֽ�������Na2CO3
B���Ʊ�����ú���У�����Ϊú�Ͳ����Ʒ�����Ӧ���Ʊ�ú���ܶȴ�ú�Ϳ���ʹ�Ƹ���������ˮ����
C���ýྻ�IJ����������Na2O2����֬��������֬��ȼ�գ�˵��CO2��H2O��Na2O2�ķ�Ӧ�Ƿ��ȷ�Ӧ
D���Ƴ��ڱ�¶�ڿ����еIJ�����Na2CO3��ԭ���������������ɵ�Na2O��ˮ�Ͷ�����̼��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
2013��7���Ĵ�ʡ���ֵ�������ǿ�������������ؾ�������ˮ�ܵ�������Ⱦ�����������һ����Ҫ����ɫ��ˮ�������о�ˮ������˫�ع��ܡ�ij����ѧϰС��������·����Ʊ�������أ�

(1)�����������Ԫ�صĻ��ϼ�Ϊ________���Ʋ������еĻ�ѧ������__________________��
(2)��NaClO��Һ�м����ռ�����γɼ��Ի���������ĥ��������������εؼ���������Һ�У���ˮԡ�з�Ӧ1 h�����������ӷ�ӦΪ2Fe3����3ClO����10OH��===2FeO ��3Cl����5H2O����������________����KOH���뷴Ӧ�����Һ�н����Сʱ�����ã����˴ֲ�Ʒ���ù��̷����ķ�ӦΪ2KOH��Na2FeO4===K2FeO4��2NaOH�����ݸ��ֽⷴӦԭ�����������Ƶ��ܽ��________(����ڡ�����С�ڡ����ڡ�)������ص��ܽ�ȡ�
��3Cl����5H2O����������________����KOH���뷴Ӧ�����Һ�н����Сʱ�����ã����˴ֲ�Ʒ���ù��̷����ķ�ӦΪ2KOH��Na2FeO4===K2FeO4��2NaOH�����ݸ��ֽⷴӦԭ�����������Ƶ��ܽ��________(����ڡ�����С�ڡ����ڡ�)������ص��ܽ�ȡ�
(3)��ǿ������Һ�У���������ܽ��Ǹ����(KCrO2)����Ϊ�����(K2CrO4)�����ɵĸ������ܽ��ữ�õ����ظ����(K2Cr2O7)��(NH4)2Fe(SO4)2����Һ�ζ����Զ�����������Ϊָʾ��������ζ��յ�ʱ����Һ����ɫ��Ϊ����ɫ(��6�۸�ת���ɣ�3�۸�)���йط�Ӧ�����ӷ���ʽΪFeO ��CrO
��CrO ��2H2O===CrO
��2H2O===CrO ��Fe(OH)3����OH����2CrO
��Fe(OH)3����OH����2CrO ��2H��===Cr2O
��2H��===Cr2O ��H2O��Cr2O
��H2O��Cr2O ��6Fe2����14H��===2Cr3����6Fe3����7H2O��
��6Fe2����14H��===2Cr3����6Fe3����7H2O��
�ֳ�ȡ5.00 g���������Ʒ���ձ��У�������������������Һ�������Թ�����KCrO2����ַ�Ӧ��ת�Ƶ�250 mL ����ƿ�У����ݣ���ȡ25.00 mL������ϡ�����ữ����0.100 0 mol��L��1 (NH4)2Fe(SO4)2����Һ�ζ������ı���Һ33.33 mL����������ʵ���Ƶõ���Ʒ�и�����ص�����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��NA���������ӵ�������������������ȷ����
A��1mol/L�İ�ˮ���������ˮ��Ϻ�������仯����������ҺŨ�ȴ���0��5mol/L
B��1mol Na2O2��������ˮ��ȫ��Ӧ��ת�Ƶĵ�����Ϊ2NA
C���ڱ�״���£�22��4L������22��4L���������е�ԭ������Ϊ2NA
D��28g��ϩ�ͻ����飨C3H6���Ļ�����庬�е�ԭ������Ϊ3NA
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com