
��12�֣������������ͬ��NaOH��Һ�У��ֱ�ͨ��CO2���壬��ַ�Ӧ����������ҺA��B�зֱ����1mol/L�����ᣬ�������������mL�������CO2����������L���Ĺ�ϵ��ͼ��ʾ������д���пհף�
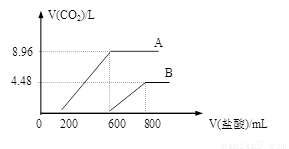
������NaOH��ҺA��B�����ʵ���Ũ��֮��Ϊ ��ͨ��CO2��������֮���ǣ���ͬ������ ��
�������ᷴӦǰ��A��Һ�е������� �������ʵ���֮���� ��
�������ᷴӦǰ��B��Һ�е������� �������ʵ���֮���� ��
 �п������п��Ծ����ϵ�д�
�п������п��Ծ����ϵ�д� ��������״Ԫ��ϵ�д�
��������״Ԫ��ϵ�д� �ƸԿ�����ҵ��ϵ�д�
�ƸԿ�����ҵ��ϵ�д� ��Ԫ����ĩ��ϰ�ȷ��ϵ�д�
��Ԫ����ĩ��ϰ�ȷ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| c(B) |
| c(OH-) |
| c(OH-) |
| c(H+) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2012?����ģ�⣩��Դ��ȱ���������ٵ��ش����⣮�״���һ�ֿ�������Դ�����й㷺�Ŀ�����Ӧ��ǰ������˼״�����Ϊ21���͵�����ȼ�ϣ�
��2012?����ģ�⣩��Դ��ȱ���������ٵ��ش����⣮�״���һ�ֿ�������Դ�����й㷺�Ŀ�����Ӧ��ǰ������˼״�����Ϊ21���͵�����ȼ�ϣ�| 3 |
| 2 |
| 3 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�����ʡ�߶���ѧ�����п��Ի�ѧ�������Ծ� ���ͣ������
 ���¶ȸ���40��ʱ��ͭ��ƽ���ܽ��������ŷ�Ӧ�¶����߶��½�������Ҫԭ����
��
���¶ȸ���40��ʱ��ͭ��ƽ���ܽ��������ŷ�Ӧ�¶����߶��½�������Ҫԭ����
��
�����ᴿ���CuSO4��Һ�м���һ������Na2SO3��NaCl��Һ�����ȣ�����CuCl�������Ʊ�CuCl�����ӷ���ʽ�� ��
28. (9��)����һ�仰������������ֵ��ǰ��ΪM������ΪN��M��N�Ĺ�ϵ��A��B��C��D��ѡ��
A. M>N B. M<N C. M=N D. ���Ƚ�
����ͬ�¶��£�1L 1mol/L ��NH4Cl��Һ�е�NH4��������2 L 0.5mol��L��1NH4Cl��Һ��NH4���ĸ����� ��
����ͬ�¶��£�pHֵΪ12���ռ���Һ��ˮ�ĵ���Ⱥ�pHֵΪ12��CH3COONa��Һ��ˮ�ĵ���ȣ� ��
����������ʱ�ı���ʯ��ˮ��һ�����µ�50�棻��һ�ݼ�������CaO���ָ������£�����Һ�е�c(Ca2+)�� ��
�ȳ��������ݵ�Ũ�ȵĴ�����Һ�����ڶ��������¶ȣ�����Һ��c(HCO3��)�� ��
�ɽ�pHֵΪ2�Ĵ�������ᶼϡ����ͬ��������ϡ��Һ��pHֵ�� ��
�ʳ�����0.1mol/L��CH3COOH��0.1mol/LCH3COONa�������Ϻ���Һ��c(Na+)��c(CH3COO��)�� ��
��ͬ�¶��£�0.1mol/LFeCl3��Һ��Fe3+ˮ��ٷ�����0.01mol��L��1FeCl3��Һ��Fe3+ ��ˮ��ٷ��ʣ� ��
��������ijǿ���ijǿ����Һ�������Ϻ���Һ��pHֵΪ7��ԭ����Һ��ԭ����Һ�����ʵ���Ũ�ȣ� ��
��PHֵ��ͬ�Ĵ�������ᣬ�ֱ�������ˮϡ����ԭ����M����N����ϡ�ͺ�����Һ��PHֵ��Ȼ��ͬ�� ��M��N�Ĺ�ϵ�ǣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���¶ȸ���40��ʱ��ͭ��ƽ���ܽ��������ŷ�Ӧ�¶����߶��½�������Ҫԭ���� ��
�����ᴿ���CuSO4��Һ�м���һ������Na2SO3��NaCl��Һ�����ȣ�����CuCl�������Ʊ�CuCl�����ӷ���ʽ�� ��
28. (9��)����һ�仰������������ֵ��ǰ��ΪM������ΪN��M��N�Ĺ�ϵ��A��B��C��D��ѡ��
A. M>N B.M<N C.M=N D. ���Ƚ�
����ͬ�¶��£�1L 1mol/L ��NH4Cl��Һ�е�NH4��������2 L 0.5mol��L��1NH4Cl��Һ��NH4���ĸ����� ��
����ͬ�¶��£�pHֵΪ12���ռ���Һ��ˮ�ĵ���Ⱥ�pHֵΪ12��CH3COONa��Һ��ˮ�ĵ���ȣ� ��
����������ʱ�ı���ʯ��ˮ��һ�����µ�50�棻��һ�ݼ�������CaO���ָ������£�����Һ�е�c(Ca2+)�� ��
�ȳ��������ݵ�Ũ�ȵĴ�����Һ�����ڶ��������¶ȣ�����Һ��c(HCO3��)�� ��
�ɽ�pHֵΪ2�Ĵ�������ᶼϡ����ͬ��������ϡ��Һ��pHֵ�� ��
�ʳ�����0.1mol/L��CH3COOH��0.1mol/LCH3COONa�������Ϻ���Һ��c(Na+)��c(CH3COO��)�� ��
��ͬ�¶��£�0.1mol/LFeCl3��Һ��Fe3+ˮ��ٷ�����0.01mol��L��1FeCl3��Һ��Fe3+ ��ˮ��ٷ��ʣ� ��
��������ijǿ���ijǿ����Һ�������Ϻ���Һ��pHֵΪ7��ԭ����Һ��ԭ����Һ�����ʵ���Ũ�ȣ� ��
��PHֵ��ͬ�Ĵ�������ᣬ�ֱ�������ˮϡ����ԭ����M����N����ϡ�ͺ�����Һ��PHֵ��Ȼ��ͬ�� ��M��N�Ĺ�ϵ�ǣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ�������и߶���ѧ�����п��Ի�ѧ�������Ծ� ���ͣ������
 ���¶ȸ���40��ʱ��ͭ��ƽ���ܽ��������ŷ�Ӧ�¶����߶��½�������Ҫԭ���� ��
���¶ȸ���40��ʱ��ͭ��ƽ���ܽ��������ŷ�Ӧ�¶����߶��½�������Ҫԭ���� ��
�����ᴿ���CuSO4��Һ�м���һ������Na2SO3��NaCl��Һ�����ȣ�����CuCl�������Ʊ�CuCl�����ӷ���ʽ�� ��
28.(9��)����һ�仰������������ֵ��ǰ��ΪM������ΪN��M��N�Ĺ�ϵ��A��B��C��D��ѡ��
A. M>N B. M<N C. M="N" D. ���Ƚ�
����ͬ�¶��£�1L 1mol/L ��NH4Cl��Һ�е�NH4��������2 L 0.5mol��L��1NH4Cl��Һ��NH4���ĸ����� ��
����ͬ�¶��£�pHֵΪ12���ռ���Һ��ˮ�ĵ���Ⱥ�pHֵΪ12��CH3COONa��Һ��ˮ�ĵ���ȣ� ��
����������ʱ�ı���ʯ��ˮ��һ�����µ�50�棻��һ�ݼ�������CaO���ָ������£�����Һ�е�c(Ca2+)�� ��
�ȳ��������ݵ�Ũ�ȵĴ�����Һ�����ڶ��������¶ȣ�����Һ��c(HCO3��)�� ��
�ɽ�pHֵΪ2�Ĵ�������ᶼϡ����ͬ��������ϡ��Һ��pHֵ�� ��
�ʳ�����0.1mol/L��CH3COOH��0.1mol/LCH3COONa�������Ϻ���Һ��c(Na+)��c(CH3COO��)�� ��
��ͬ �¶��£�0.1mol/LFeCl3��Һ��Fe3+ˮ��ٷ�����0.01mol��L��1FeCl3��Һ��Fe3+��ˮ��ٷ��ʣ� ��
�¶��£�0.1mol/LFeCl3��Һ��Fe3+ˮ��ٷ�����0.01mol��L��1FeCl3��Һ��Fe3+��ˮ��ٷ��ʣ� ��
��������ijǿ���ijǿ����Һ�������Ϻ���Һ��pHֵΪ7�� ԭ����Һ��ԭ����Һ�����ʵ���Ũ�ȣ� ��
ԭ����Һ��ԭ����Һ�����ʵ���Ũ�ȣ� ��
��PHֵ��ͬ�Ĵ�������ᣬ�ֱ�������ˮϡ����ԭ����M����N����ϡ�ͺ�����Һ��PHֵ��Ȼ��ͬ����M��N�Ĺ�ϵ�ǣ� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com