
(��)��ʢ������ձ���ע����������ˮ�����ò��������裬ʹ���Ͼ��ȡ�
(��)��������ȴ����_________ע��________mL��________�С�
(��)��________________��ȡ1.19g/cm3�����ʵ���������Ϊ37%��Ũ����Լ___________mLע���ձ��С�
(��)����������ˮϴ���ձ���������2��3�Σ�����Һһ��ע������ƿ�С�
(��)������ƿС�ļ�����ˮ��Һ��ӽ��̶�______________��������____________������ˮ��ʹ��Һ��_____________��ƿ���̶����С�
(��)���ñ��IJ�������ҡ�ȣ���ת�����Լ�ƿ�����ϱ�ǩ��
(2)�ش������й����⣺
����Ͳ��ȡһ�����Һ����㵹���ձ��У���Ͳ�ڲ�����Һ��Ӧ���������������ʹ��ɵ���ҺŨ����α仯��
�ڽ����ձ����ܽ��ϡ�ͺõ�Һ��ת��������ƿ�У�Ӧ������������δ������������ʹ�����ҺŨ�������仯��
�۶���ʱ�������Ӷ��̶ȣ���ʹ��ɵ���Һ��Ũ�������������أ�
����������ƿ�ǽ�������ҡ�ȣ����ƿ���Ϳ̶������ϻ����һЩ��ҺʹҺ�潵�ͣ������õ�����ҺŨ�Ȼ������仯��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
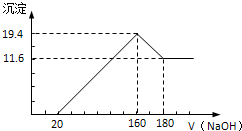 ������������250mL 0.1mol/L��Na2CO3��Һ����ղ���ش��������⣺
������������250mL 0.1mol/L��Na2CO3��Һ����ղ���ش��������⣺| ʵ��Ӧ��Na2CO3����/g | Ӧѡ������ƿ�Ĺ��/mL | ������ƿ���Ҫ���������� |
| 2.7g | 250ml | ���������ձ�����ͷ�ι� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ����һ��ѧ��ѧ����� ���ͣ�058
����Լ0.1mol/L������Һ500mL���뽫����˳��(1��2��3����)��д����������ں����������ʵ����������ơ������������ʵ���ţ�
(����)��ʢ������ձ���ע������������ˮ�����ò��������裬ʹ���Ͼ��ȣ�
(����)��������ȴ����________ע��________mL��________�У�
(����)����ȡ�ܶ�Ϊ1.19g/ �����ʵ���������Ϊ37%��Ũ����Լ________mLע���ձ��У�
�����ʵ���������Ϊ37%��Ũ����Լ________mLע���ձ��У�
(����)����������ˮϴ���ձ���������2��3�Σ�����Һһ��ע������ƿ�У�
(����)������ƿ��С�ļ�����ˮ����Һ��ӽ��̶ȴ�������________������ˮ��ʹ��Һ��________��ƿ���̶����У�
(����)���ñ��IJ�������ҡ�ȣ���ת�����Լ�ƿ�����ϱ�ǩ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�058
��1������Լ0.1mol/L������Һ500mL���뽫����˳����д����������ں����������ʵ����������ơ������������ʵ����֡�
(��)��ʢ������ձ���ע����������ˮ�����ò��������裬ʹ���Ͼ��ȡ�
(��)��������ȴ����_________ע��________mL��________�С�
(��)��________________��ȡ1.19g/cm3�����ʵ���������Ϊ37%��Ũ����Լ___________mLע���ձ��С�
(��)����������ˮϴ���ձ���������2��3�Σ�����Һһ��ע������ƿ�С�
(��)������ƿС�ļ�����ˮ��Һ��ӽ��̶�______________��������____________������ˮ��ʹ��Һ��_____________��ƿ���̶����С�
(��)���ñ��IJ�������ҡ�ȣ���ת�����Լ�ƿ�����ϱ�ǩ��
(2)�ش������й����⣺
����Ͳ��ȡһ�����Һ����㵹���ձ��У���Ͳ�ڲ�����Һ��Ӧ���������������ʹ��ɵ���ҺŨ����α仯��
�ڽ����ձ����ܽ��ϡ�ͺõ�Һ��ת��������ƿ�У�Ӧ������������δ������������ʹ�����ҺŨ�������仯��
�۶���ʱ�������Ӷ��̶ȣ���ʹ��ɵ���Һ��Ũ�������������أ�
����������ƿ�ǽ�������ҡ�ȣ����ƿ���Ϳ̶������ϻ����һЩ��ҺʹҺ�潵�ͣ������õ�����ҺŨ�Ȼ������仯��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com