
µ—È “–Ë“™O£Æ1 mol£ØL NaOH»Ð“∫450 mL∫Õ0£Æ5mol£Øl¡ÚÀ·»Ð“∫480 mL°£∏˘æð’‚¡Ω÷÷»Ð“∫µƒ≈‰÷∆«Èøˆªÿ¥œ¬¡–Œ £∫
(1)»ÁÕºÀ˘ 浃“«∆˜÷–≈‰÷∆»Ð“∫–Ë“™µƒ « (ÃÓ◊÷∫≈)£¨≈‰÷∆…œ ˆ»Ð“∫ªπ–Ë”√µΩµƒ≤£¡ß“«∆˜ « (ÃÓ“«∆˜√˚≥∆)°£

(2)œ¬¡–≤Ÿ◊˜÷–£¨ «»ð¡ø∆øÀ˘æþ±∏µƒπ¶ƒÐ”– °£
A£Æ≈‰÷∆“ª∂®Ãª˝◊º»∑≈®∂»µƒ±Í◊º»Ð“∫
B£Æ÷¸¥Ê»Ð“∫
C£Æ≈‰÷∆»Œ“‚≈®∂»∫Õê˝µƒ±Í◊º»Ð“∫
D£Æœ° Õƒ≥“ª≈®∂»µƒ»Ð“∫
E£Æ¡ø»°“ª∂®Ãª˝µƒ“∫ÃÂ
(3)∏˘æðº∆À„”√Õ–≈ÃÃÏ∆Ω≥∆»°NaOHµƒ÷ ¡øŒ™ g°£‘⁄ µ—È÷–∆‰À˚≤Ÿ◊˜æ˘’˝»∑£¨»Ù∂®»ð ±∏© ”øÃ∂»œþ£¨‘ÚÀ˘µ√»Ð“∫≈®∂» (ÃÓ°∞¥Û”⁄°±°∞µ»”⁄°±ªÚ°∞–°”⁄°±)O£Æ1 mol£Øl°£»ÙNaOH»Ð“∫‘⁄◊™“∆÷¡»ð¡ø∆ø ±£¨»˜¬‰¡À…Ÿ–Ì£¨‘Ú–Ë“™»Á∫Œ≤Ÿ◊˜£∫ °£(4)∏˘æðº∆À„µ√÷™£¨À˘–Ë÷ ¡ø∑÷ ˝Œ™98£•°¢√Ð∂»Œ™l.84 g£Øcm3µƒ≈®¡ÚÀ·µƒÃª˝Œ™ ml°£»Áπ˚ µ—È “”–10 mL£¨15 mL£¨20 mL¡øÕ≤£¨”¶—°”√ mLµƒ¡øÕ≤◊Ó∫√°£≈‰÷∆π˝≥Ã÷––Ëœ»‘⁄…’±≠÷–Ω´≈®¡ÚÀ·Ω¯––œ° Õ£¨œ° Õ ±≤Ÿ◊˜∑Ω∑® «
(5)œ¬¡–≤Ÿ◊˜ª· π≈‰÷∆µƒNaOH»Ð“∫≈®∂»∆´µÕ µƒ «°£
A£Æ”√¬À÷Ω≥∆¡øNaOH
B£Æ—°”√µƒ»ð¡ø∆øƒ⁄”–…Ÿ¡ø’Ù¡ÛÀÆ
C£Æ∂®»ð“°‘»∫Û£¨“∫√Êœ¬Ωµ£¨”÷º”ÀÆ÷¡øÃ∂»œþ
D£Æ’˚∏ˆ≈‰÷∆π˝≥Ã÷–£¨»ð¡ø∆ø≤ª’Òµ¥

| ƒÍº∂ | ∏þ÷–øŒ≥à | ƒÍº∂ | ≥ı÷–øŒ≥à |
| ∏þ“ª | ∏þ“ª√‚∑—øŒ≥ÃÕ∆ºˆ£° | ≥ı“ª | ≥ı“ª√‚∑—øŒ≥ÃÕ∆ºˆ£° |
| ∏þ∂˛ | ∏þ∂˛√‚∑—øŒ≥ÃÕ∆ºˆ£° | ≥ı∂˛ | ≥ı∂˛√‚∑—øŒ≥ÃÕ∆ºˆ£° |
| ∏þ»˝ | ∏þ»˝√‚∑—øŒ≥ÃÕ∆ºˆ£° | ≥ı»˝ | ≥ı»˝√‚∑—øŒ≥ÃÕ∆ºˆ£° |
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫ –գ∫
œ¬¡–∑÷◊”÷–À˘”–‘≠◊”∂º¬˙◊„◊ÓÕ‚≤„8µÁ◊”Ω·π𵃠«£® £©
A π‚∆¯£®COCl2£© B ¡˘∑˙ªØ¡Ú C ∂˛∑˙ªØÎØ D »˝∑˙ªØ≈
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫ –գ∫
ÕÍ≥…œ¬¡–∑¥”¶µƒ¿Î◊”∑Ω≥Ã Ω£∫
£®1£©œÚNaHSO4»Ð“∫÷–÷µŒº”»ÎBa(OH)2»Ð“∫£¨ π‘≠»Ð“∫÷–µƒSO42°™«°∫√ÕÍ»´≥¡µÌ£∫
£®2£©œÚ√˜∑ػГ∫÷–÷µŒº”»ÎBa(OH)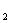 »Ð“∫£¨µ±Al3+«°∫√»´≤ø≥¡µÌ ±£∫
»Ð“∫£¨µ±Al3+«°∫√»´≤ø≥¡µÌ ±£∫
£®3£©œÚCa£®HC03£©2»Ð“∫÷–º”»Îπ˝¡øµƒNaOH»Ð“∫£∫
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫ –գ∫
œ¬¡–∏˜—°œÓ÷–µƒ¡Ω∏ˆ∑¥”¶,ø…”√Õ¨“ª∏ˆ¿Î◊”∑Ω≥Ã Ω±Ì æµƒ « ( )
| —°œÓ | ¢Ò | ¢Ú |
| A | Ba(OH)2»Ð“∫”Îπ˝¡øNaHCO3»Ð“∫ªÏ∫œ | NaOH»Ð“∫”Îπ˝¡øNaHCO3»Ð“∫ªÏ∫œ |
| B | …Ÿ¡øSO2Õ®»ÎBa(OH)2»Ð“∫÷– | π˝¡øSO2Õ®»ÎBa(OH)2»Ð“∫÷– |
| C | BaCl2»Ð“∫”ÎNa2SO3»Ð“∫ªÏ∫œ | Ba(OH)2»Ð“∫”ÎH2SO3»Ð“∫ªÏ∫œ |
| D | …Ÿ¡ø∞±ÀƵŒ»ÎAlCl3»Ð“∫÷– | …Ÿ¡øAlCl3»Ð“∫µŒ»Î∞±ÀÆ÷– |
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫ –գ∫
œ¬¡–π˝≥Ã√ª”–∑¢…˙ªØ—ß∑¥”¶µƒ «
A£Æ”√ªÓ–‘Ãø»•≥˝±˘œ‰÷–µƒ“ÏŒ∂
B£Æ”√»»ºÓÀÆ«Â≥˝¥∂æþ…œ≤–¡Ùµƒ”ÕŒ€
C£Æ”√Ω˛≈ðπ˝∏þ√ÃÀ·ºÿ»Ð“∫µƒπË‘ÂÕ¡±£œ ÀÆπ˚
D£Æ”√∫¨πËΩ∫°¢Ã˙∑€µƒÕ∏∆¯–°¥¸”Î ≥∆∑“ª∆√Ð∑‚∞¸◊∞
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫ –գ∫
œ¬¡–πÿ”⁄‘™Àÿ÷Ð∆⁄±Ì∫Õ‘™Àÿ÷Ð∆⁄¬…µƒÀµ∑®≤ª’˝»∑µƒ «(°°°°)
A£Æ¥”∑˙µΩµ‚£¨µ•÷ µƒ—ıªØ–‘÷Ω•ºı»ı£¨«‚ªØŒÔµƒªπ‘≠–‘÷Ω•‘ˆ«ø
B£Æµ»ŒÔ÷ µƒ¡øµƒƒ∆‘≠◊”±»¬¡‘≠◊” ß»•µƒµÁ◊” ˝…Ÿ£¨À˘“‘ƒ∆±»¬¡µƒªπ‘≠–‘»ı
C£Æ¥”ƒ∆µΩ¬»£¨◊Ó∏þº€—ıªØŒÔµƒÀƪ،ԺӖ‘÷Ω•ºı»ı£¨À·–‘÷Ω•‘ˆ«ø
D£Æ—ı”Ρڌ™Õ¨÷˜◊‘™Àÿ£¨—ı±»¡Úµƒ‘≠◊”∞Îæ∂–°£¨—ı∆¯±»¡Úµƒ—ıªØ–‘«ø
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫ –գ∫
œ¬¡–”–πÿ»»ªØ—ß∑Ω≥Ã Ωµƒ±Ì æº∞Àµ∑®’˝»∑µƒ «(°°°°)
A£Æ“—÷™2H2(g)+O2(g)£Ω2H2O(g)°° °˜H=£≠483.6kJ/mol£¨‘Ú«‚∆¯µƒ»º…’»»Œ™241.8kJ/mol°°
B£Æ“—÷™C( ؃´£¨s)£ΩC(Ω∏’ Ø£¨s) °˜H>0£¨‘ÚΩ∏’ ر» ؃´Œ»∂®
C£Æ∫¨20.0g NaOHµƒœ°»Ð“∫”Îœ°¡ÚÀ·ÕÍ»´÷–∫Õ£¨∑≈≥ˆ28.7kJµƒ»»¡ø£¨‘Ú±Ì æ∏√∑¥”¶÷–∫Õ»»µƒ»»ªØ—ß∑Ω≥Ã ΩŒ™£∫NaOH(aq)+1/2H2SO4 (aq)£ΩNaCl(aq)+H2O(l) °˜H=£≠57.4kJ/mol
D£Æ“—÷™I2(g)+ H2(g)£Ω2HI(g) °˜H1£¨°°I2(s)+ H2(g)£Ω2HI(g )°° °˜H2°°‘Ú°˜H1>°˜H2
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫ –գ∫
Õ¨∑÷“ÏππÃÂæþ”–£∫¢Ÿ œýÕ¨µƒœý∂‘∑÷◊”÷ ¡ø ¢⁄ œýÕ¨µƒ∑÷◊” Ω ¢€ œýÕ¨µƒ◊ÓºÚ Ω
¢Ð œýÕ¨µƒŒÔ¿Ì–‘÷ °°¢ð œýÕ¨µƒªØ—ß–‘÷
A£Æ¢Ÿ¢⁄¢€ B£Æ¢Ÿ¢⁄¢Ð C£Æ¢Ÿ¢⁄¢ð D£Æ¢⁄¢€¢ð
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫ –գ∫
œÚ∫¨”–Fe2£´°¢I£≠°¢Br£≠µƒ»Ð“∫÷–Õ®»Î ¡ø¬»∆¯£¨»Ð“∫÷–∏˜÷÷¿Î◊”µƒŒÔ÷ µƒ¡ø±‰ªØ»ÁÕºÀ˘ æ°£”–πÿÀµ∑®≤ª’˝»∑µƒ «( )
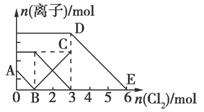
A£Æœþ∂ŒBC¥˙±ÌFe3£´ŒÔ÷ µƒ¡øµƒ±‰ªØ«Èøˆ
B£Æ‘≠ªÏ∫œ»Ð“∫÷–n(FeBr2)=3mol
C£Æµ±Õ®»Î2molCl2 ±£¨»Ð“∫÷–“—∑¢…˙µƒ¿Î◊”∑¥”¶Œ™£∫2Fe2£´+2I£≠+2Cl2=2Fe3£´+I2+4Cl£≠
D£Æ‘≠»Ð“∫÷–n(Fe2£´)£∫n(I£≠)£∫n(Br£≠)=3°√1°√2
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
∞Ÿ∂»÷¬–≈ - ¡∑œ∞≤·¡–±Ì - ‘¡–±Ì
∫˛±± °ª•¡™Õ¯Œ•∑®∫Õ≤ª¡º–≈œ¢æŸ±®∆Ωî | Õ¯…œ”–∫¶–≈œ¢æŸ±®◊®«¯ | µÁ–≈’©∆≠柱®◊®«¯ | …Ê¿˙ ∑–ÈŒÞ÷˜“”–∫¶–≈œ¢æŸ±®◊®«¯ | …Ê∆Û«÷»®æŸ±®◊®«¯
Œ•∑®∫Õ≤ª¡º–≈œ¢æŸ±®µÁª∞£∫027-86699610 柱®” œ‰£∫58377363@163.com