
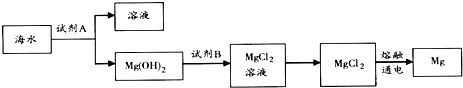
���� ��1���Ӻ�ˮ�Ʊ����ʵ�ԭ����֪������������ǽ���������Ҫ���û�ѧ��Ӧ����ȡ����ʳ�ο���������ԭ������ˮ��������ԭ�����õ���
��2����������������������������Ϊ�嵥�ʣ�
��3����ˮ�м�����ʯ�ҳ���þ���ӹ��˵õ�������þ�����������Լ�BΪ���ᣬ������þ�ܽ�Ϊ�Ȼ�þ��Һ��Ũ�������ᾧ�õ��Ȼ�þ���壬�缫�����Ȼ�þ�õ�����þ��������
�ٹ�ҵ�ϳ����ڳ���Mg2+���Լ�A���������ƣ�Mg��OH��2������������MgCl2����Һ��
�ڵ�������Ȼ�þ�õ�����þ��������
��4�������չ���ʱ���õ���Ҫ����������������
�������Ӻ������⽫����������Ϊ���ʵ⣻
�۵��CCl4��Һ����ɫ��
��� �⣺��1��A����ˮ����Ԫ��Ϊ�����ӣ��ǵ�ⱥ��ʳ��ˮ���������Ȼ��Ƶõ������������˻�ѧ�仯����A����
B���Ѻ�ˮ������ȷ������Եõ���ˮ������Ҫ��ѧ�仯����B��ȷ��
C����ⱥ���Ȼ�����Һ�õ��ռ���������������������ڻ�ѧ�仯����C����
D���Ѻ�ˮ��̫����ɹ������ˮ�ֺ�ʳ�Σ�����Ҫ��ѧ�仯���ܹ��Ӻ�ˮ�л�ã���D��ȷ��
�ʴ�Ϊ��BD��
��2���Ӻ�ˮ����ȡ�����Ҫ��������Ũ���ĺ�ˮ��ͨ�����������������������÷�Ӧ�����ӷ���ʽ�ǣ�Cl2+2Br-=Br2+2Cl-��
�ʴ�Ϊ��Cl2+2Br-=Br2+2Cl-��
��3����ˮ�м�����ʯ�ҳ���þ���ӹ��˵õ�������þ�����������Լ�BΪ���ᣬ������þ�ܽ�Ϊ�Ȼ�þ��Һ��Ũ�������ᾧ�õ��Ȼ�þ���壬�缫�����Ȼ�þ�õ�����þ��������
�ٹ�ҵ�ϳ����ڳ���Mg2+���Լ�A���������ƣ�Mg��OH��2������������MgCl2����Һ�����ӷ���ʽΪ��Mg��OH��2+2H+=Mg2++2H2O��
�ʴ�Ϊ����ʯ�ң�Mg��OH��2+2H+=Mg2++2H2O��
�ڵ�������Ȼ�þ�õ�����þ����������Ӧ�Ļ�ѧ����ʽΪ��MgCl2$\frac{\underline{\;ͨ��\;}}{\;}$Mg+Cl2����
�ʴ�Ϊ��MgCl2$\frac{\underline{\;ͨ��\;}}{\;}$Mg+Cl2����
��4�������չ���ʱ���õ���Ҫ�����������������ʴ�Ϊ��������
�ڼ��������Ӻ��������������Ϊ����������������ת��Ϊ���ʵ⣬���ӷ���ʽΪ2H++2I-+H2O2�TI2+2H2O���ʴ�Ϊ��2H++2I-+H2O2�TI2+2H2O��
�۵��CCl4��Һ����ɫ���ʴ�Ϊ���ϣ�
���� ���⿼���˺�ˮ��Դ���ۺ����ã����ݻ�ѧ��Ӧ�ĸ������ͼ�����ʵ��������������֪������ͼ��ÿһ���ķ�Ӧ������д��Ӧ�Ļ�ѧ����ʽ��֪����������ij�����������Ŀ�ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | O3��O2��Ϊͬλ�� | |
| B�� | O3��O2���ת���������仯 | |
| C�� | ��ͬ״���£������O3��O2������ͬ������ | |
| D�� | O3��O2�����Ը�ǿ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
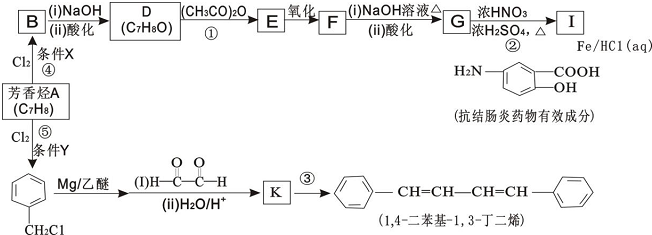
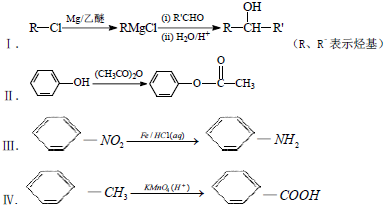
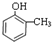
 ��
��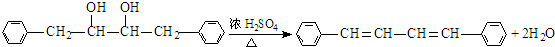 ��
��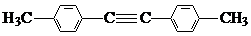 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Cr2O72-��CrO42-��Ũ����ͬ | B�� | 2v����Cr2O72-��=v����CrO42-�� | ||
| C�� | ��Һ����ɫ���� | D�� | ��Һ��pH���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

 ��
���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com