
ijʵ��С��ֱ���ͼ1��2װ�òⶨij�ָ�Ƭ��̼��Ƶĺ������г�װ������ȥ��
�ṩ���Լ�����ϸ�ĸ�Ƭ��ĩ(��Ƭ�е������ɷֲ������ᷴӦ)��2 mol��L��1���ᡢ5����NaOH��Һ������Na2CO3��Һ������NaHCO3��Һ������ˮ��
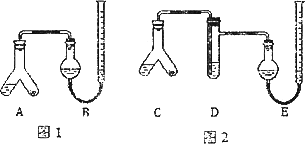
ʵ����̣�
�������װ�õ������ԣ�
����A��C���ұ���0.25 g��Ƭ��ĩ�������3 mL��2 mol��L��1���ᣬ�������ӣ���B��E�о����뱥��NaHCO3��Һ����ͼ��ʾ�����������ܶ�����
��A��C��б��ʹҺ��������ϣ�ʵ���������ȴ����������ܶ��������B���ռ���������Ϊ41.90 mL��E���ռ������������Ϊ39.20 mL(�������������������Ϊ��״���µ����)��
�ش��������⣺
(1)���м��ͼ1װ�������Ե�����________��
(2)A�з�����Ӧ�����ӷ���ʽΪ________��D�м����Լ�Ϊ________��D��������________��
(3)ʵ��ǰ��������Һ����ͬһˮƽ���ϣ�������ʱ�ҹܵ�Һ�������ܵ�Һ�棬Ӧ���еIJ�����________��
(4)ͼ2ʵ�����ø�Ƭ�е�̼��Ƶ���������Ϊ________��ͼ1ʵ���ͼ2ʵ�����ø�Ƭ�е�̼��ƺ���ƫ�ߣ����������ʽ��ʾƫ�ߵ�ԭ��________��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2011?��������ģ��ijʵ��С��ֱ���ͼ1��2װ�òⶨij�ָ�Ƭ��̼��Ƶĺ������г�װ������ȥ��
��2011?��������ģ��ijʵ��С��ֱ���ͼ1��2װ�òⶨij�ָ�Ƭ��̼��Ƶĺ������г�װ������ȥ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ijʵ��С��ֱ���ͼ1��2װ�òⶨij�ָ�Ƭ��̼��Ƶĺ������г�װ������ȥ��
ijʵ��С��ֱ���ͼ1��2װ�òⶨij�ָ�Ƭ��̼��Ƶĺ������г�װ������ȥ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(14��)ijʵ��С��ֱ���ͼ1��2װ�òⶨij�ָ�Ƭ��̼��Ƶĺ������г�װ������ȥ���ṩ���Լ�:��ϸ�ĸ�Ƭ��ĩ (��Ƭ�е������ɷֲ������ᷴӦ)��![]() ���ᡢ5%NaOH��Һ������
���ᡢ5%NaOH��Һ������![]() ��Һ������
��Һ������![]() ��Һ������ˮ��
��Һ������ˮ��
ʵ�����:
��.�������װ�õ������ԡ�
��.��A��C���ұ���0.25g��Ƭ��ĩ�������3mL![]() ���ᣬ�������ӡ���B��E�о����뱥��
���ᣬ�������ӡ���B��E�о����뱥��![]() ��Һ,��ͼ��ʾ�����������ܶ�����
��Һ,��ͼ��ʾ�����������ܶ�����
��.��A��C��б��ʹҺ��������ϣ�ʵ���������ȴ����������ܶ��������B���ռ���������Ϊ41.90mL��E���ռ������������Ϊ39.20mL(�������������������Ϊ��״���µ��������
�ش���������:
(1)���м��ͼ1װ�������Եķ�����
��
��2��A�з�����Ӧ�����ӷ���ʽΪ ��
D�м����Լ�Ϊ ��D�������� ��
��3��ʵ��ǰ��������Һ����ͬһˮƽ���ϣ�������ʱ�ҹܵ�Һ�������ܵ�Һ�棬Ӧ���еIJ�����
��
��4��ͼ2ʵ�����ø�Ƭ�е�̼��Ƶ���������Ϊ ;ͼ1ʵ���ͼ 2ʵ�����ø�Ƭ�е�̼��ƺ���ƫ�ߣ����������ʽ��ʾƫ�ߵ�ԭ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��2011ѧ����������23�и߶���ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ�ʵ����
��8�֣�ijʵ��С��ֱ���ͼ1��2װ�òⶨij�ָ�Ƭ��̼��Ƶĺ������г�װ������ȥ��
�ṩ���Լ�����ϸ�ĸ�Ƭ��ĩ����Ƭ�е������ɷֲ������ᷴӦ����2mol/L ���ᡢ5%NaOH��Һ������Na2CO3��Һ������NaHCO3��Һ������ˮ��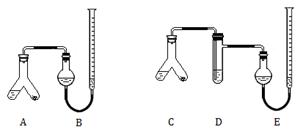
ͼ1 ͼ2
ʵ����̣�
I���������װ�õ������ԡ�
II����A��C���ұ���0.25 g��Ƭ��ĩ�������3 mL 2mol/L���ᣬ�������ӡ���B��E�о����뱥��NaHCO3��Һ����ͼ��ʾ�����������ܶ�����
��A��C��б��ʹҺ��������ϣ�ʵ���������ȴ����������ܶ�������� B���ռ���������Ϊ41.90 mL��E���ռ������������Ϊ39.20 mL���������������������Ϊ��״���µ��������
�ش��������⣺
��1��I�м��ͼ1װ�������Եķ����� ��
��2��A�з�����Ӧ�����ӷ���ʽΪ ��D�м�����Լ�Ϊ ��
��3��ʵ��ǰ��������Һ����ͬһˮƽ���ϣ�������ʱ�ҹܵ�Һ�������ܵ�Һ�棬
Ӧ���еIJ����� ��
��4��ͼ2ʵ�����ø�Ƭ�е�̼��Ƶ���������Ϊ ��ͼ1ʵ���ͼ2ʵ�����ø�Ƭ�е�̼��ƺ���ƫ�ߣ������ӷ���ʽ��ʾƫ�ߵ�ԭ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012����������23�и߶���ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ�ʵ����
��8�֣�ijʵ��С��ֱ���ͼ1��2װ�òⶨij�ָ�Ƭ��̼��Ƶĺ������г�װ������ȥ��
�ṩ���Լ�����ϸ�ĸ�Ƭ��ĩ����Ƭ�е������ɷֲ������ᷴӦ����2mol/L ���ᡢ5%NaOH��Һ������Na2CO3��Һ������NaHCO3��Һ������ˮ��

ͼ1 ͼ2
ʵ����̣�
I���������װ�õ������ԡ�
II����A��C���ұ���0.25 g��Ƭ��ĩ�������3 mL 2mol/L���ᣬ�������ӡ���B��E�о����뱥��NaHCO3��Һ����ͼ��ʾ�����������ܶ�����
��A��C��б��ʹҺ��������ϣ�ʵ���������ȴ����������ܶ�������� B���ռ���������Ϊ41.90 mL��E���ռ������������Ϊ39.20 mL���������������������Ϊ��״���µ��������
�ش��������⣺
��1��I�м��ͼ1װ�������Եķ����� ��
��2��A�з�����Ӧ�����ӷ���ʽΪ ��D�м�����Լ�Ϊ ��
��3��ʵ��ǰ��������Һ����ͬһˮƽ���ϣ�������ʱ�ҹܵ�Һ�������ܵ�Һ�棬
Ӧ���еIJ����� ��
��4��ͼ2ʵ�����ø�Ƭ�е�̼��Ƶ���������Ϊ ��ͼ1ʵ���ͼ2ʵ�����ø�Ƭ�е�̼��ƺ���ƫ�ߣ������ӷ���ʽ��ʾƫ�ߵ�ԭ�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com