
£Ø¹²9·Ö£©A”¢B”¢C”¢D”¢EĪåÖÖĪļÖŹµÄŃęÉ«¶¼³Ź×ĻÉ«(Ķø¹żĄ¶É«īܲ£Į§)£¬A”¢BÓėĖ®·“Ó¦¶¼ÓŠĘųĢå·Å³ö£¬AÓėĖ®·“Ó¦·Å³öµÄĘųĢå¾ßÓŠ»¹ŌŠŌ£¬BÓėĖ®·“Ó¦·Å³öµÄĘųĢå¾ßÓŠŃõ»ÆŠŌ£¬Ķ¬Ź±¶¼Éś³ÉČÜŅŗC.CÓėŹŹĮæµÄCO2·“Ӧɜ³ÉD£¬Óė¹żĮæµÄCO2·“Ӧɜ³ÉE.E¼ÓČČÄÜÉś³ÉD.ŹŌĶʶĻ£ŗ
¢ÅA£ŗ_____________£»B£ŗ_____________£»C£ŗ_____________£»D£ŗ_____________£»E£ŗ_____________(Š“»ÆѧŹ½)
¢Ę°“ŅŖĒ󊓳öÓŠ¹Ų·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ
BÓėĖ®·“Ó¦µÄ»Æѧ·½³ĢŹ½
E¼ÓČČÉś³ÉDµÄ»Æѧ·½³ĢŹ½

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| H2SO4(ÅØ) |
| 170”ę |
| ŅŅ“¼ | 1£¬2-¶žäåŅŅĶé | ŅŅĆŃ | äå | |
| É«”¢Ģ¬ | ĪŽÉ«ŅŗĢå | ĪŽÉ«ŅŗĢå | ĪŽÉ«ŅŗĢå | Éīŗģ×ŲÉ«ŅŗĢå |
| ĆܶČ/£Øg?cm-3£© | 0.79 | 2.2 | 0.71 | 3.1 |
| ·Šµć/”ę | 78.5 | 132 | 34.6 | 59.47 |
| ČŪµć/”ę | -130 | 9 | -116 | -7.25 |
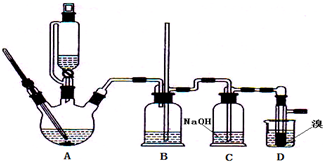
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğŗŚĮś½Ź”ĖēĄāĻŲµŚŅ»ÖŠŃ§ø߶žÉĻŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
£Ø¹²10·Ö£©(1)”¢£Ø2·Ö£©ĻąĶ¬ĪļÖŹµÄĮæÅضČĻĀµÄø÷ČÜŅŗ£ŗNaX”¢NaY”¢NaZ£¬ĘäPHÖµŅĄ“ĪĪŖ8”¢9”¢10£¬ŌņHX”¢HY”¢HZµÄĖįŠŌÓÉĒæµ½ČõµÄĖ³ŠņĪŖ:
(2)”¢£Ø2·Ö£©½«AlCl3ČÜÓŚĖ®ŗ󣬾¼ÓČČÕō·¢”¢ÕōøÉ£¬×ĘÉÕ£¬×īŗóĖłµĆ°×É«¹ĢĢåµÄÖ÷ŅŖ³É·ÖŹĒ
(3)”¢£Ø2·Ö£© °Ńa”¢b”¢c”¢dĖÄÖÖ½šŹōʬ½žÅŻŌŚĻ”ĮņĖįÖŠ£¬ÓƵ¼ĻßĮ½Į½ĻąĮ¬£¬æÉŅŌ×é³Éø÷ÖÖŌµē³Ų”£Čōa bĻąĮ¬£¬aĪŖøŗ¼«£»c dĻąĮ¬£¬cĪŖøŗ¼«£»a cĻąĮ¬£¬cĪŖÕż¼«£»b dĻąĮ¬£¬bĪŖÕż¼«£¬ŌņĖÄÖÖ½šŹōµÄ»ī¶ÆŠŌÓÉĒæµ½ČõĖ³ŠņĪŖ£ŗ
(4)£Ø2·Ö£©Š“³öNa2CO3ČÜÓŚĖ®ŗóµÄµēĄė·½³ĢŹ½£ŗ _____________________________________________________
(5)£Ø2·Ö£©Š“³öĀČ»ÆĢśČÜŅŗĖ®½ā·“Ó¦µÄĄė×Ó·½³ĢŹ½____________________________________________________
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
(7·Ö) »ÆŗĻĪļA”¢BŗĶC»„ĪŖĶ¬·ÖŅģ¹¹Ģ唣ĖüĆĒµÄŌŖĖŲ·ÖĪöŹż¾ŻĪŖ£ŗĢ¼ 92.3%, Ēā 7.7%”£1 mol AŌŚŃõĘųÖŠ³ä·ÖČ¼ÉÕ²śÉś179.2 L¶žŃõ»ÆĢ¼£Ø±ź×¼×“æö£©”£AŹĒ·¼Ļć»ÆŗĻĪļ£¬·Ö×ÓÖŠĖłÓŠµÄŌ×Ó¹²Ę½Ćę£»BŹĒ¾ßÓŠĮ½øöÖ§Į“µÄĮ“ד»ÆŗĻĪļ£¬·Ö×ÓÖŠÖ»ÓŠĮ½ÖÖ²»Ķ¬»Æѧ»·¾³µÄĒāŌ×Ó£¬Å¼¼«¾ŲµČÓŚĮć£»CŹĒĶéĢž£¬·Ö×ÓÖŠĢ¼Ō×ӵĻÆѧ»·¾³ĶźČ«ĻąĶ¬”£
9-1 Š“³öA”¢BŗĶCµÄ·Ö×ÓŹ½”£
9-2 »³öA”¢BŗĶCµÄ½į¹¹¼ņŹ½”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com