
�� 6 �֣��ش��������⣺
��1����ӦA(g)+B(g)![]() C(g)+D(g)�����е������仯����ͼ��ʾ���жϸ÷�Ӧ��H 0 ���������������������ȷ��������
C(g)+D(g)�����е������仯����ͼ��ʾ���жϸ÷�Ӧ��H 0 ���������������������ȷ��������
��2����Al2O3��Ni������̬���ᷢ�����з�Ӧ��
����(g)=CO (g)+ H2O (g)�� ��H1= + 34.0 kJ/mol
����(g)=CO2 (g)+ H2(g) ��H2=��7.0 kJ/mol
�����ķ���ʽΪ ���ڸ������£���̬CO2 ����̬H2������̬CO����̬H2O���Ȼ�ѧ����ʽΪ ��
��3������ƽ�����ʢ��ǿ��ԭ��Һ̬�£�N2H4����ǿ������Һ̬˫��ˮ��H2O2����
����0.4molҺ̬�º�0.8molҺ̬H2O2��Ϸ�Ӧ�����ɵ�����ˮ�������ų�256.7kJ
������(�൱��25�桢101 kPa�²�õ�����)����Ӧ���Ȼ�ѧ����ʽΪ
��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��ʡ����һ�и߶�ģ���⻯ѧ�Ծ� ���ͣ������
�� 6 �֣��ش��������⣺
��1����ӦA(g)+B(g) C(g)+D(g)�����е������仯����ͼ��ʾ���жϸ÷�Ӧ��H 0 ���������������������ȷ��������
C(g)+D(g)�����е������仯����ͼ��ʾ���жϸ÷�Ӧ��H 0 ���������������������ȷ��������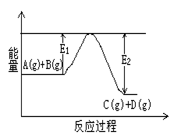
��2����Al2O3��Ni������̬���ᷢ�����з�Ӧ��
����(g)=" CO" (g)+ H2O (g)�� ��H1=" +" 34.0 kJ/mol
����(g)= CO2 (g)+ H2(g) ��H2= ��7.0 kJ/mol
�����ķ���ʽΪ ���ڸ������£���̬CO2����̬H2������̬CO����̬H2 O���Ȼ�ѧ����ʽΪ ��
O���Ȼ�ѧ����ʽΪ ��
��3������ƽ�����ʢ��ǿ��ԭ��Һ̬�£�N2H4����ǿ�� ����Һ̬˫��ˮ��H2O2����
����Һ̬˫��ˮ��H2O2����
����0.4molҺ̬�º�0.8mol Һ̬H2O2��Ϸ�Ӧ�����ɵ�����ˮ�������ų�256.7kJ
������(�൱��25�桢101 kPa�²�õ�����)����Ӧ���Ȼ�ѧ����ʽΪ
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��6���㽭����У�߶���ĩ���Ի�ѧ���� ���ͣ������
(6��)�Իش���������(�¶Ⱦ�Ϊ25��ʱ)��
��1����0. 2mol / L HB��Һ��0.1 mol / LMOH��Һ�������ϣ���û����Һ��pH=4,������Һ����ˮ�������c��H����__ __0. 2 mol / L HB��Һ����ˮ�������c��H�����������������������="��" )
(2)pH=13��Ba(OH)2��Һa L��pH=3��HCl��Һb L��ϡ������û����Һ�����ԣ�
��a��b= ��
��3�������˺���������̬ϵͳ�У�����Ҫ�����ȥ��CO2����Ҫ���ṩ�����O2��ij�ֵ绯ѧװ�ÿ�ʵ������ת����2 CO2��2 CO��O2��CO������ȼ�ϡ���֪�÷�Ӧ��������ӦΪ��4OH����4e����O2����2H2O��������ӦʽΪ________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�긣������������ѧ��һ��ѧ�����п����Ŀƻ�ѧ�Ծ����������� ���ͣ������
(6��)��Ҫ�ش��������⡣
��1������ȼ��������Ȼ����ˮ��������Ʊ���������Ҫ��ȼ�ɷ��Ǽ��飬д������ȼ�յĻ�ѧ����ʽ ��
��2����������Al��OH)3��һ������θ�ᣨ�����ᣩ�����ҩ����û�ѧ����ʽ��ʾ�÷�Ӧ��ԭ���� ��
��3������ͷʱ�������������з���С�մ�ʹ����ͷ���ɣ�ʳ����������ɿ�,��д����ԭ��??????????? ?????????????��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ɽ��ʡ�߶�ģ���⻯ѧ�Ծ� ���ͣ������
�� 6 �֣��ش��������⣺
��1����ӦA(g)+B(g) C(g)+D(g)�����е������仯����ͼ��ʾ���жϸ÷�Ӧ��H 0 ���������������������ȷ��������
C(g)+D(g)�����е������仯����ͼ��ʾ���жϸ÷�Ӧ��H 0 ���������������������ȷ��������
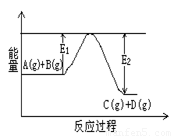
��2����Al2O3��Ni������̬���ᷢ�����з�Ӧ��
����(g)= CO (g)+ H2O (g)�� ��H1= + 34.0 kJ/mol
����(g)= CO2 (g)+ H2(g) ��H2= ��7.0 kJ/mol
�����ķ���ʽΪ ���ڸ������£���̬CO2 ����̬H2 ������̬CO����̬H2O���Ȼ�ѧ����ʽΪ ��
��3������ƽ�����ʢ��ǿ��ԭ��Һ̬�£�N2H4����ǿ������Һ̬˫��ˮ��H2O2����
����0.4molҺ̬�º�0.8mol Һ̬H2O2��Ϸ�Ӧ�����ɵ�����ˮ�������ų�256.7kJ
������(�൱��25�桢101 kPa�²�õ�����)����Ӧ���Ȼ�ѧ����ʽΪ
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��6���㽭����У�߶���ĩ���Ի�ѧ���� ���ͣ������
(6��)�Իش���������(�¶Ⱦ�Ϊ25��ʱ)��
��1����0. 2mol / L HB��Һ��0.1 mol / LMOH��Һ�������ϣ���û����Һ��pH=4,������Һ����ˮ�������c��H����__ __0. 2 mol / L HB��Һ����ˮ�������c��H�����������������������=�� )
(2)pH=13��Ba(OH)2��Һa L��pH=3��HCl��Һb L��ϡ������û����Һ�����ԣ�
��a��b= ��
��3�������˺���������̬ϵͳ�У�����Ҫ�����ȥ��CO2����Ҫ���ṩ�����O2��ij�ֵ绯ѧװ�ÿ�ʵ������ת����2 CO2��2 CO��O2��CO������ȼ�ϡ���֪�÷�Ӧ��������ӦΪ��4OH����4e����O2����2H2O��������ӦʽΪ________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com