
£Ø16£©Ä³æĪĶā»ī¶ÆŠ”×éÉč¼ĘĮĖŅŌĻĀŹµŃé·½°øŃéÖ¤CuÓėÅØĻõĖį·“Ó¦µÄ¹ż³ĢÖŠæÉÄܲśÉśNO”£Ę䏵ŃéĮ÷³ĢĶ¼ČēĻĀ£ŗ
£Ø1£©²ā¶ØĻõĖįµÄĪļÖŹµÄĮæ·“Ó¦½įŹųŗ󣬓ÓĻĀĶ¼B×°ÖĆÖŠĖłµĆ100mLČÜŅŗÖŠČ”³ö25.00mLČÜŅŗ£¬ÓĆ0.1mol”¤L-1µÄNaOHČÜŅŗµĪ¶Ø£¬ÓĆ·ÓĢŖ×÷ÖøŹ¾¼Į£¬µĪ¶ØĒ°ŗóµÄµĪ¶Ø¹ÜÖŠŅŗĆęµÄĪ»ÖĆČēÓŅÉĻĶ¼ĖłŹ¾”£ŌŚBČŻĘ÷ÖŠÉś³ÉĻõĖįµÄĪļÖŹµÄĮæĪŖ mol”£
£Ø2£©²ā¶ØNOµÄĢå»ż
¢Ł“ÓÉĻĶ¼ĖłŹ¾µÄ×°ÖĆÖŠ£¬ÄćČĻĪŖӦєÓĆ ×°ÖĆ½ųŠŠCuÓėÅØĻõĖį·“Ó¦ŹµŃé£¬Ń”ÓƵĥķÓÉŹĒ ”£
¢ŚŃ”ÓĆÉĻĶ¼ĖłŹ¾ŅĒĘ÷×éŗĻŅ»Ģ×æÉÓĆĄ“Ķź³ÉŹµŃé²¢²ā¶ØÉś³ÉNOĢå»żµÄ×°ÖĆ£¬ĘäŗĻĄķµÄĮ¬½ÓĖ³ŠņŹĒ£ØĢīø÷µ¼¹ÜæŚ±ąŗÅ£© ”£
¢ŪŌŚ²ā¶ØNOµÄĢå»żŹ±£¬ČōĮæĶ²ÖŠĖ®µÄŅŗĆę±Č¼ÆĘųĘæµÄŅŗĆęŅŖµĶ£¬“ĖŹ±Ó¦½«ĮæĶ²µÄĪ»ÖĆ £Ø”°ĻĀ½µ”±»ņ”°Éżøß”±£©£¬ŅŌ±£Ö¤ĮæĶ²ÖŠµÄŅŗĆęÓė¼ÆĘųĘæÖŠµÄŅŗĆę³ÖĘ½”£
£Ø3£©ĘųĢå³É·Ö·ÖĪö£ŗČōŹµŃé²āµĆNOµÄĢå»żĪŖ112.0mL£ØŅŃÕŪĖćµ½±ź×¼×“æö£©£¬ŌņCuÓėÅØĻõĖį·“Ó¦µÄ¹ż³ĢÖŠ £ØĢī”°ÓŠ”±»ņ”°Ć»ÓŠ”±£©NO²śÉś£¬×÷“ĖÅŠ¶ĻµÄŅĄ¾ŻŹĒ ”£
£Ø4£©ŹµŃéĒ°£¬ÓĆĶŠÅĢĢģĘ½³ĘČ”µÄĶʬÖĮÉŁÓ¦ĪŖ g”£
£Ø1£©0.008 £Ø2£©¢ŁA£»ŅņĪŖA×°ÖĆæÉŅŌĶØČėN2½«×°ÖĆÖŠµÄæÕĘųÅž”£¬·ĄÖ¹NO±»æÕĘųÖŠO2Ńõ»Æ
¢Ś123547 ¢ŪÉżøß £Ø3£©ÓŠ£»ŅņĪŖNO2ÓėĖ®·“Ӧɜ³ÉµÄNOµÄĢå»żŠ”ÓŚŹÕ¼Æµ½µÄNOµÄĢå»ż£Ø89.6£¼112.0£© £Ø4£©0.5
½āĪöŹŌĢā·ÖĪö£ŗ£Ø1£©BČŻĘ÷ÖŠŹĒ¶žŃõ»ÆµŖŗĶĖ®·“Ӧɜ³ÉĻõĖįŗĶŅ»Ńõ»ÆµŖ£»100mLČÜŅŗÖŠČ”³ö25.00mLČÜŅŗ£¬ÓĆ0.1mol?L-1µÄNaOHČÜŅŗµĪ¶Ø£¬ÓĆ·ÓĢŖ×÷ÖøŹ¾¼Į£¬ÖÕµćŹ±£¬ĻūŗÄĒāŃõ»ÆÄĘČÜŅŗµÄĢå»żĪŖ20.4ml-0.4ml£½20ml£¬ĖłŅŌÉś³ÉĻõĖį25.00mLČÜŅŗÖŠŗ¬ÓŠ0.02L”Į0.1mol/L£½0.002mol£¬ŌņBČŻĘ÷ÖŠÉś³ÉĻõĖįµÄĪļÖŹµÄĮæĪŖ0.008mol£»
£Ø2£©¢ŁŅ»Ńõ»ÆµŖĘųĢåŅ×±»ŃõĘųŃõ»ÆÉś³É¶žŃõ»ÆµŖ¶ŌŃéÖ¤²śÉśøÉČÅ£¬ADĻą±ČA×°ÖĆĄūÓƵŖĘųæÉŅŌ°Ń×°ÖĆÖŠµÄæÕĘųÅž»£»
¢ŚÓĆA½ųŠŠĶŗĶÅØĻõĖįµÄ·“Ó¦£¬ÓĆĖ®ĪüŹÕÉś³ÉµÄ¶žŃõ»ÆµŖĘųĢ壬µ¼Ęų¹Ü³¤½ų¶Ģ³ö£¬æÉŅŌÓĆÅÅĖ®ĮæĘų·Ø²ā¶ØŅ»Ńõ»ÆµŖĘųĢåµÄĢå»ż£¬ÅÅĖ®¼ÆĘųĘæµ¼Ęų¹ÜÓ¦¶Ģ½ų³¤³ö£¬Į¬½ÓĖ³ŠņĪŖ£ŗ123547£»
¢Ū¶ĮŹżÖ®Ē°Ó¦±£³ÖÄŚĶāŃ¹ĒæĻąĶ¬£¬»Öø“µ½ŹŅĪĀĻĀ¶ĮČ”ĮæĶ²ÖŠŅŗĢåµÄĢå»ż£¬ČōĮæĶ²ÖŠĖ®µÄŅŗĆę±Č¼ÆĘųĘæµÄŅŗĆęŅŖµĶ£¬“ĖŹ±Ó¦½«ĮæĶ²µÄĪ»ÖĆÉżøߣ¬ŅŌ±£Ö¤ĮæĶ²ÖŠµÄŅŗĆęÓė¼ÆĘųĘæÖŠµÄŅŗĆę³ÖĘ½£»
£Ø3£©ŹµŃé²āµĆNOµÄĢå»żĪŖ112.0mL£ØŅŃÕŪĖćµ½±ź×¼×“æö£©£¬ŅĄ¾ŻÉĻŹö¼ĘĖćµĆµ½CuŗĶÅØĻõĖįÉś³É¶žŃõ»ÆµŖĘųĢåĪļÖŹµÄĮæĪŖ0.012mol£¬ŅĄ¾Ż3NO2+H2O=2HNO3+NOæÉÖŖŅ»Ńõ»ÆµŖĘųĢåĪļÖŹµÄĮæĪŖ0.004mol£¬±ź×¼×“æöĻĀĢå»żĪŖ0.004”Į22.4L/mol=0.0896L=89.6ml£¼112.0LæÉÖŖCuŗĶĻõĖį·“Ӧɜ³ÉŅ»Ńõ»ÆµŖĘųĢ壻
£Ø4£©ÓÉ3NO2+H2O=2HNO3+NOæÉÖŖNOĪļÖŹµÄĮæĪŖ0.004mol£¬½įŗĻ3Cu+8HNO3=3Cu£ØNO3£©2+2NO”ü+4H2OæÉÖŖ£¬ŠčŅŖCuĪŖ0.008”Į64g/mol=0.512g”Ö0.5g”£
æ¼µć£ŗæ¼²éŠŌÖŹŹµŃé·½°øµÄÉč¼Ę”¢»Æѧ¼ĘĖćµČ



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
øß“æ¹čÉś²śĮ÷³ĢČēĻĀ£ŗ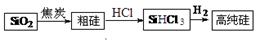
£Ø1£©ÓÉSiO2ÖĘ“Ö¹čµÄ»Æѧ·½³ĢŹ½ŹĒ £¬øĆ·“Ó¦²»ÄÜĖµĆ÷Ģ¼µÄ·Ē½šŹōŠŌĒæÓŚ¹č£¬ŌŅņŹĒ £¬ĒėŠ“³öŅ»øöÄÜĖµĆ÷Ģ¼µÄ·Ē½šŹōŠŌĒæÓŚ¹čµÄ»Æѧ·½³ĢŹ½ ”£
£Ø2£©900”ęŅŌÉĻ£¬H2ÓėSiHCl3·¢Éś·“Ó¦£ŗSiHCl3(g)+ H2(g) Si(s) + 3HCl(g) ¦¤H£¾0”£½«Ņ»¶ØĮæµÄ·“Ó¦ĪļĶØČė¹Ģ¶ØČŻ»żµÄĆܱÕČŻĘ÷ÖŠ½ųŠŠ·“Ó¦”£ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ £ØĢī×ÖÄø£©”£
Si(s) + 3HCl(g) ¦¤H£¾0”£½«Ņ»¶ØĮæµÄ·“Ó¦ĪļĶØČė¹Ģ¶ØČŻ»żµÄĆܱÕČŻĘ÷ÖŠ½ųŠŠ·“Ó¦”£ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ £ØĢī×ÖÄø£©”£
a£®ŌŚŗćĪĀĢõ¼žĻĀ£¬ČōČŻĘ÷ÄŚŃ¹Ēæ²»±ä£¬ŌņøĆ·“Ó¦Ņ»¶Ø“ļµ½»ÆŃ§Ę½ŗāדĢ¬
b£®Ōö“óSiHCl3µÄÓĆĮ棬æÉĢįøßSiHCl3µÄĘ½ŗā×Ŗ»ÆĀŹ
c£®ÉżøßĪĀ¶ČæɼÓæģ·“Ó¦ĖŁĀŹ£¬ĒŅĢįøß¹čµÄ²śĀŹ
£Ø3£©øĆĮ÷³ĢÖŠæÉŅŌŃ»·Ź¹ÓƵÄĪļÖŹŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĪŹ“šĢā
(20·Ö)ijŠ£»ÆѧŹµŃéŠĖȤŠ”×éĪŖĮĖĢ½¾æŌŚŹµŃéŹŅÖʱøCl2µÄ¹ż³ĢÖŠÓŠĖ®ÕōĘųŗĶHCl»Ó·¢³öĄ“£¬Ķ¬Ź±Ö¤Ć÷Cl2µÄijŠ©ŠŌÖŹ£¬¼×Ķ¬Ń§Éč¼ĘĮĖČēĶ¼ĖłŹ¾µÄŹµŃé×°ÖĆ£ØÖ§³ÅÓƵÄĢś¼ÜĢØŹ”ĀŌ£©£¬°“ŅŖĒó»Ų“šĪŹĢā£ŗ
£Ø1£©ĻĀĮŠ·½·ØÖŠ£¬æÉÖʵƵÄÕżČ·×éŗĻŹĒ__________”£
¢ŁMnO2ŗĶÅØŃĪĖį»ģŗĻ¹²ČČ£» ¢ŚMnO2”¢NaClŗĶÅØĮņĖį»ģŗĻ¹²ČČ£ŗ
¢ŪNaClOŗĶÅØŃĪĖį»ģŗĻ£» ¢ÜK2Cr2O7ŗĶÅØŃĪĖį»ģŗĻ£ŗ
¢ŻKClO3ŗĶÅØŃĪĖį»ģŗĻ¹²ČČ£» ¢ŽKMnO4ŗĶÅØŃĪĖį»ģŗĻ”£
A£®¢Ł¢Ś¢ŽB£®¢Ś¢Ü¢ŽC£®¢Ł¢Ü¢Ž D£®Č«²ææÉŅŌ
£Ø2£©Š“³öŹµŃéŹŅÖĘČ”Cl2µÄĄė×Ó·½³ĢŹ½____________”£
£Ø3£©ČōÓĆŗ¬ÓŠ0.2 mol HClµÄÅØŃĪĖįÓė×ćĮæµÄMnO2·“Ó¦ÖʵĆCl2µÄĢå»ż£Ø±źæöĻĀ£©×ÜŹĒŠ”ÓŚ1.12LµÄŌŅņŹĒ_________________________________________”£
£Ø4£©¢Ł×°ÖĆBµÄ×÷ÓĆŹĒ__________________________________”£
¢Ś×°ÖĆCŗĶD³öĻֵIJ»Ķ¬ĻÖĻóĖµĆ÷µÄĪŹĢāŹĒ________________________”£
¢Ū×°ÖĆEµÄ×÷ÓĆŹĒ_____________________”£
£Ø5£©ŅŅĶ¬Ń§ČĻĪŖ¼×Ķ¬Ń§µÄŹµŃéӊȱĻŻ£¬²»ÄÜČ·Ļń×īÖÕĶØČėAgNO3ČÜŅŗÖŠµÄĘųĢåÖ»ÓŠŅ»ÖÖ”£ĪŖĮĖČ·±£ŹµŃé½įĀŪµÄæÉææŠŌ£¬Ö¤Ć÷×īÖÕĶØČėAgNO3ČÜŅŗÖŠµÄĘųĢåÖ»ÓŠŅ»ÖÖ£¬ŅŅĶ¬Ń§Ģį³öÓ¦øĆŌŚ×°ÖĆ__________Óė________Ö®¼ä£ØĢī×°ÖĆ×ÖÄøŠņŗÅ£©Ōö¼ÓŅ»øö×°ÖĆ£¬Ōö¼Ó×°ÖĆĄļĆęµÄŹŌ¼ĮæÉĪŖ____________”£
A£®ŹŖČóµÄµķ·ŪKIŹŌÖ½B£®ĒāŃõ»ÆÄĘČÜŅŗ
C£®ŹŖČóµÄŗģÉ«²¼Ģõ D£®±„ŗĶµÄŹ³ŃĪĖ®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¼ĘĖćĢā
°Ń19£®2 gµÄCu·ÅČė×ćĮæµÄĻ”ĻõĖįÖŠ£¬Ī¢ČČÖĮCuĶźČ«·“Ó¦”£
ŅŃÖŖ£ŗ3Cu + 8HNO3(Ļ”) = 3Cu(NO3)2 +2NO”ü+ 4H2OĒó£ŗ
£Ø1£©²Ī¼Ó·“Ó¦µÄĻõĖįµÄĪļÖŹµÄĮ棻
£Ø2£©±»»¹ŌµÄĻõĖįµÄÖŹĮ棻
£Ø3£©Éś³ÉµÄNOŌŚ±ź×¼×“æöĻĀµÄĢå»ż”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¼ĘĖćĢā
ĻõĖįŹĒŅ»ÖÖÖŲŅŖµÄ¹¤ŅµŌĮĻ£¬¹¤ŅµÖĘĻõĖįµÄ¹Ų¼üŹĒ°±µÄ“ß»ÆŃõ»Æ£¬ÓėĻõĖį¹¤ŅµĻą¹ŲµÄ¹ż³ĢÖŠ²śÉśµÄµŖŃõ»ÆĪļµÄ“¦ĄķÓėÓ¦ÓĆŅ²ŹĒæĘѧъ¾æµÄČČµć”£
I£®Ķ¼10”¢Ķ¼11·Ö±šŹĒŹµŃéŹŅÄ£ÄāŗĻ³É°±¼°°±“ß»ÆŃõ»ÆµÄ×°ÖĆ
£Ø1£©µŖĘųŗĶĒāĘųĶعżĶ¼10×°ÖĆ£¬øĆ×°ÖĆÖŠÅØĮņĖįµÄ×÷ÓĆŹĒæŲÖĘĘųĢåĮ÷ĖŁŗĶ ”£
£Ø2£©ÓĆĶ¼11×°ÖĆĪüŹÕŅ»¶ĪŹ±¼ä°±ŗó£¬ŌŁĶØČėæÕĘų£¬Ķ¬Ź±½«ŅŃ¾¼ÓČČµÄ²¬Ėæ²åČėŅŅ×°ÖƵÄ׶ŠĪĘæÄŚ£¬²¬Ėæ±£³ÖŗģČȵÄŌŅņŹĒ £¬Š“³öøĆ×°ÖĆÖŠ°±Ńõ»ÆµÄ»Æѧ·½³ĢŹ½ ”£·“Ó¦½įŹųŗó׶ŠĪĘæÄŚµÄČÜŅŗÖŠŗ¬ÓŠH£«”¢OH£”¢ Ąė×Ó”¢ Ąė×Ó”£
II£®ĻĀĮŠÓŠ¹ŲĻõĖįŹĀŹµµÄ½āŹĶŗĻĄķµÄŹĒ
| A£®ÅØĻõĖįĶس£±£“ęŌŚ×ŲÉ«µÄŹŌ¼ĮĘæÖŠ£¬ĖµĆ÷ÅØĻõĖį²»ĪČ¶Ø |
| B£®×ćĮæĢśÓėĻ”ĻõĖį·“Ó¦ŗóČÜŅŗ³ŹĒ³ĀĢÉ«£¬ĖµĆ÷Ļ”ĻõĖį²»ÄÜŃõ»ÆŃĒĢśĄė×Ó |
| C£®²»ÓĆÅØĻõĖįÓėĶŠ¼·“Ó¦Ą“ÖĘČ”ĻõĖįĶ£¬ĖµĆ÷ÅØĻõĖį¾ßÓŠ»Ó·¢ŠŌ |
| D£®²»ÓĆŠæÓėĻ”ĻõĖį·“Ó¦ÖĘČ”ĒāĘų£¬ĖµĆ÷Ļ”ĻõĖįÄܽ«Šæ¶Ū»Æ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
ijŠ”×éĶ¬Ń§ÓĆĻĀĶ¼×°ÖĆ½ųŠŠŹµŃéŃŠ¾æ(a”¢b”¢c±ķŹ¾Ö¹Ė®¼Š)£®ĒėĘĄ¼Ū»ņĶźÉĘĘä·½°ø£ŗ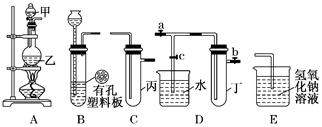
½«×°ÖĆA”¢C”¢EĻąĮ¬½Ó£¬ÓĆMnO2ŗĶÅØŃĪĖįÖĘČ”ĀČĘų£¬Ēė»Ų“š£ŗ
¢Ł£®ĀČŌŖĖŲŌŚÖÜĘŚ±ķÖŠµÄĪ»ÖĆĪŖ ”£
¢Ś£®AÖŠ·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ___ _”£
¢Ū£®EÖŠĒāŃõ»ÆÄĘČÜŅŗµÄ×÷ÓĆ__________ ”£
ŌŚCÖŠ¼ÓČėŹŹĮæµÄĖ®æÉÖʵĆĀČĖ®£®½«ĖłµĆĀČĖ®·Ö³ÉĮ½·Ż½ųŠŠŹµŃ飬Ęä²Ł×÷”¢ĻÖĻóŗĶ½įĀŪĪŖ£ŗ
| ŹµŃéŠņŗÅ | ŹµŃé²Ł×÷ | ĻÖĻó | ½įĀŪ |
| ¢ń | ½«ĀČĖ®µĪČėĘ·ŗģČÜŅŗ | Ę·ŗģČÜŅŗĶŹÉ« | ĀČĘųÓŠĘư׊Ō |
| ¢ņ | ĀČĖ®ÖŠ¼ÓČėĢ¼ĖįĒāÄĘ·ŪÄ© | ÓŠĪŽÉ«ĘųÅŻ²śÉś | ĀČĘųÓėĖ®·“Ó¦µÄ²śĪļ¾ßÓŠĖįŠŌ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
ĪŖŃŠ¾æĶÓėÅØĮņĖįµÄ·“Ó¦£¬Ä³»ÆѧŠĖȤŠ”×é½ųŠŠČēĻĀŹµŃ锣
ŹµŃé¢ń£ŗ·“Ó¦²śĪļµÄ¶ØŠŌĢ½¾æ”£
ŹµŃé×°ÖĆČēĶ¼ĖłŹ¾£ŗ£Ø¹Ģ¶Ø×°ÖĆŅŃĀŌČ„£©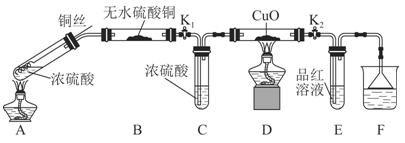
£Ø1£©AÖŠ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø2£©FÉÕ±ÖŠµÄČÜŅŗĶس£ŹĒ ”£
£Ø3£©ŹµŃé¹ż³ĢÖŠ£¬ÄÜÖ¤Ć÷ÅØĮņĖįÖŠĮņŌŖĖŲµÄŃõ»ÆŠŌĒæÓŚĒāŌŖĖŲµÄĻÖĻóŹĒ
ӣ
£Ø4£©ŹµŃé½įŹųŗó£¬Ö¤Ć÷A×°ÖĆŹŌ¹ÜÖŠ·“Ó¦ĖłµĆ²śĪļŹĒ·ńŗ¬ÓŠĶĄė×ӵIJŁ×÷·½·ØŹĒ ”£
£Ø5£©ĪŖĖµĆ÷ÅØĮņĖįÖŠµÄĖ®ŹĒ·ńÓ°ĻģB×°ÖĆĻÖĻóµÄÅŠ¶Ļ£¬»¹Šė½ųŠŠŅ»“ĪŹµŃ锣ŹµŃé·½°øĪŖ ”£
ŹµŃé¢ņ£ŗ·“Ó¦²śĪļµÄ¶ØĮæĢ½¾æ
£Ø6£©ŌŚĶÓėÅØĮņĖį·“Ó¦µÄ¹ż³ĢÖŠ£¬·¢ĻÖÓŠŗŚÉ«ĪļÖŹ³öĻÖ£¬¾²éŌÄĪÄĻ×»ńµĆĻĀĮŠ×ŹĮĻ”£
׏ĮĻ1£ŗ
| ĮņĖį/mol”¤L£1 | ŗŚÉ«ĪļÖŹ³öĻÖµÄĪĀ¶Č/”ę | ŗŚÉ«ĪļÖŹĻūŹ§µÄĪĀ¶Č/”ę |
| 15 | Ō¼150 | Ō¼236 |
| 16 | Ō¼140 | Ō¼250 |
| 18 | Ō¼120 | ²»ĻūŹ§ |
 £«I2===S4O
£«I2===S4O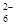 £«2I££©
£«2I££©²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
ČēĶ¼ĖłŹ¾ŹĒŌŚŹµŃéŹŅ½ųŠŠ°±ĘųæģĖŁÖʱøÓėŠŌÖŹŹµŃéµÄ×éŗĻ×°ÖĆ£¬²æ·Ö¹Ģ¶Ø×°ÖĆĪ“»³ö”£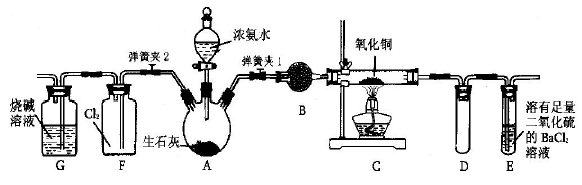
£Øl£©Š“³ö×°ÖĆAÖŠĖł·¢ÉśµÄ»Æѧ·“Ó¦
£Ø2£©×°ÖĆBÖŠŹ¢·ÅŹŌ¼ĮŹĒ
£Ø3£©µćČ¼C“¦¾Ę¾«µĘ£¬¹Ų±ÕµÆ»É¼Š2£¬“ņæŖµÆ»É¼Š1£¬“Ó·ÖŅŗĀ©¶··Å³öÅØ°±Ė®ÖĮ½žĆ»ÉÕĘæÖŠ¹ĢĢåŗó¹Ų±Õ·ÖŅŗĀ©¶·£¬ÉŌŗóʬæĢ£¬×°ÖĆCÖŠŗŚÉ«¹ĢĢåÖš½„±äŗģ£¬×°ÖĆEÖŠČÜŅŗĄļ³öĻÖ“óĮæĘųÅŻ£¬Ķ¬Ź±²ś
Éś £ØĢīŠ“·“Ó¦ĻÖĻ󣩣»“ÓEÖŠŅŻ³öŅŗĆęµÄĘųĢåæÉŅŌÖ±½ÓÅÅČėæÕĘų£¬ĒėŠ“³öŌŚCÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½
£Ø4£©ŅŃÖŖCu2OŹĒŅ»ÖÖŗģÉ«¹ĢĢåĪļÖŹ, ŌŚøßĪĀĢõ¼žĻĀæÉÓÉCuO·Ö½āµĆµ½£ŗ4CuO£½2Cu2O+O2”ü£¬Éś³ÉµÄCu2O Ņ²Äܱ»NH3»¹Ō”£µ±CÖŠ¹ĢĢåČ«²æ±äŗģÉ«ŗ󣬹Ų±ÕµÆ»É¼Š1£¬ĀżĀżŅĘæŖ¾Ę¾«µĘ£¬“żĄäČ“ŗ󣬳ĘĮæCÖŠ¹ĢĢåÖŹĮ攣Čō·“Ó¦Ē°¹ĢĢåÖŹĮæĪŖ16g£¬·“Ó¦ŗó³ĘÖŲ¹ĢĢåÖŹĮæ¼õÉŁ2£®4g”£Ķعż¼ĘĖćČ·¶ØøĆ¹ĢĢå²śĪļµÄ³É·ÖŹĒ £ØÓĆ»ÆѧŹ½±ķŹ¾£©
£Ø5£©ŌŚ¹Ų±ÕµÆ»É¼Š1ŗ󣬓ņæŖµÆ»É¼Š2£¬²ŠÓąĘųĢå½ųČėFÖŠ£¬ŗÜæģ·¢ĻÖ×°ÖĆFÖŠ²śÉś°×ŃĢ£¬Ķ¬Ź±·¢ĻÖGÖŠČÜŅŗŃøĖŁµ¹ĪüĮ÷ČėFÖŠ”£Š“³ö²śÉś°×ŃĢµÄ»Æѧ·½³ĢŹ½
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
ij»ÆѧŠĖȤŠ”×éÓūÉč¼ĘŹ¹ÓĆČēĻĀ×°ÖĆŃéÖ¤£ŗĶŗĶŅ»¶ØĮæµÄÅØĻõĖį·“Ó¦ÓŠŅ»Ńõ»ÆµŖ²śÉś”££Ø¼ŁÉčĘųĢåĢå»ż¾łĪŖ±ź×¼×“æö£¬µ¼Ęų¹ÜÖŠĘųĢåĢå»żŗöĀŌ²»¼Ę£¬ĒŅŗöĀŌ·“Ó¦ÖŠČÜŅŗµÄĢå»ż±ä»Æ£©ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)ŌŚĶŗĶÅØĻõĖį·“Ó¦Ē°£¬¼·Ń¹“ņĘųĒņ£¬¾A”¢B”¢CČżøö×°ÖĆŗ󣬽ųČė×°ÖĆDÖŠµÄĘųĢåŹĒ £ØĢī»ÆѧŹ½£©£¬ĶØČėøĆĘųĢåµÄÄæµÄŹĒ £»½ųŠŠ“Ė²½²Ł×÷Ź±Ó¦¹Ų±Õ £ØĢī”°K1”±”°K2”±»ņ”°K3”±£¬ĻĀĶ¬£©£¬“ņæŖ ”£
(2)Čō×°ÖĆCµÄÓ²ÖŹ²£Į§¹ÜÖŠ³öĻÖ¶ĀČū£¬Ōņ×°ÖĆBÖŠæÉÄܳöĻÖµÄĻÖĻóŹĒ ”£
(3)¹Ų±ÕK1”¢K2£¬“ņæŖK3£¬ÓÉ·ÖŅŗĀ©¶·Ļņ×°ÖĆDµÄŹŌ¹ÜÖŠµĪ¼ÓÅØĻõĖį”£“żCuŗĶÅØĻõĖį·“Ó¦½įŹųŗó£¬ŌŁĶعż·ÖŅŗĀ©¶·Ļņ×°ÖĆDµÄŹŌ¹ÜÖŠ¼ÓČėCCl4ÖĮĀś”£Ōņ×°ÖĆDµÄŹŌ¹ÜÖŠŅ»¶Ø·¢ÉśµÄĄė×Ó·“Ó¦·½³ĢŹ½ĪŖ ”£
(4)“Ó×°ÖĆEĖłµĆČÜŅŗÖŠČ”³ö25£®00 mL£¬ÓĆ0£®1000 mol/LµÄNaOHČÜŅŗ½ųŠŠÖŠŗĶ£¬µ±Ē”ŗĆ³ŹÖŠŠŌŹ±ĻūŗÄNaOHČÜŅŗ18£®00mL £¬Ōņ×°ÖĆEÖŠĖłµĆĻõĖįµÄĪļÖŹµÄĮæÅضČĪŖ ”£
(5)ŹµŃéĒ°ĮæĘų¹ÜµÄŅŗĆę¶ĮŹżĪŖ368£®50 mL£¬ŹµŃéŗóĮæĘų¹ÜµÄŅŗĆę¶ĮŹżĪŖ224£®00 mL”£ŌņÉĻŹöĶŗĶŅ»¶ØĮæµÄÅØĻõĖį·“Ó¦ÖŠ £ØĢī”°ÓŠ”±»ņ”°ĪŽ”±£©NOÉś³É£¬Š“³öĶʵ¼¹ż³Ģ ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com