
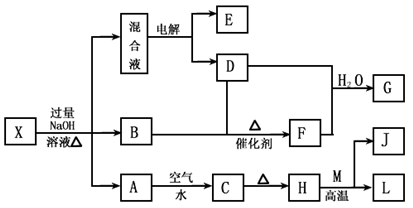
��һ������X����ˮ��ҺΪdz��ɫ���ɷ������µ�ת����ϵ�����ַ�Ӧ����������ԣ������У�B��D��E��F��Ϊ��ɫ���壬M��LΪ�����Ľ������ʣ�CΪ������ˮ�ĺ��ɫ���塣�ڻ��Һ�м���BaCl2��Һ�����ɲ�����ϡ����İ�ɫ������H��M��Ӧ�ɷų��������ȡ�
��ش��������⣺
��1��B�ĵ���ʽΪ ��
��2������Ԫ��M��ԭ�ӽṹʾ��ͼ ��
��3������X�Ļ�ѧʽΪ ��
��4�������Һʱ������ӦʽΪ ��
��5����Ҫ��д������ת����ϵ���йط�Ӧ�Ļ�ѧ����ʽ��
�� ����LԪ�صĻ��Ϸ�Ӧ ��
�� ����LԪ�ص��û���Ӧ ��
��6����֪![]()
 E������D����ȫȼ�������ȶ��Ļ�����ʱ���ų�
E������D����ȫȼ�������ȶ��Ļ�����ʱ���ų�![]() kJ��������д��Eȼ���ȵ��Ȼ�ѧ����ʽ ��
kJ��������д��Eȼ���ȵ��Ȼ�ѧ����ʽ ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ������X����ˮ��ҺΪdz��ɫ���ɷ������µ�ת����ϵ�����ַ�Ӧ����������ԣ������У�B��D��E��F��Ϊ��ɫ���壬M��LΪ�����Ľ������ʣ�CΪ������ˮ�ĺ��ɫ���塣�ڻ��Һ�м���BaCl2��Һ�����ɲ�����ϡ����İ�ɫ������H��M��Ӧ�ɷų��������ȡ�
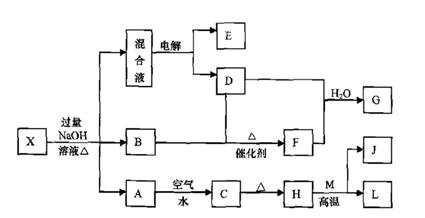
��ش��������⣺
��1��B�ĵ���ʽΪ ��
��2������Ԫ��M��ԭ�ӽṹʾ��ͼ ��
��3������X�Ļ�ѧʽΪ ��
��4�������Һʱ������ӦʽΪ ��
��5����Ҫ��д������ת����ϵ���йط�Ӧ�Ļ�ѧ����ʽ��
�� ����LԪ�صĻ��Ϸ�Ӧ ��
�� ����LԪ�ص��û���Ӧ ��
��6����֪![]()
![]() E������D����ȫȼ�������ȶ��Ļ�����ʱ���ų�
E������D����ȫȼ�������ȶ��Ļ�����ʱ���ų�![]() kJ��������д��Eȼ���ȵ��Ȼ�ѧ����ʽ ��
kJ��������д��Eȼ���ȵ��Ȼ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ��ӱ�ʡ������ѧ�ڵ�һ�ε��п��ԣ����ۣ���ѧ���� ���ͣ������
��һ������X����ˮ��ҺΪdz��ɫ���ɷ������µ�ת����ϵ�����ַ�Ӧ����������ԣ������У�B��D��E��F��Ϊ��ɫ���壬M��LΪ�����Ľ������ʣ�CΪ������ˮ�ĺ��ɫ���塣�ڻ��Һ�м���BaCl2��Һ�����ɲ�����ϡ����İ�ɫ������H��M��Ӧ�ɷų��������ȡ�
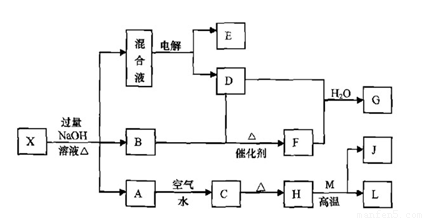
��ش��������⣺
��1��B�ĵ���ʽΪ ��
��2������Ԫ��M��ԭ�ӽṹʾ��ͼ ��
��3������X�Ļ�ѧʽΪ ��
��4�������Һʱ������ӦʽΪ ��
��5����Ҫ��д������ת����ϵ���йط�Ӧ�Ļ�ѧ����ʽ��
�� ����LԪ�صĻ��Ϸ�Ӧ ��
�� ����LԪ�ص��û���Ӧ ��
��6����֪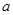
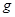 E������D����ȫȼ�������ȶ��Ļ�����ʱ���ų�
E������D����ȫȼ�������ȶ��Ļ�����ʱ���ų�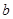 kJ��������д��Eȼ���ȵ��Ȼ�ѧ����ʽ
��
kJ��������д��Eȼ���ȵ��Ȼ�ѧ����ʽ
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���㽭ʡģ���� ���ͣ��ƶ���
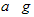 E������D����ȫȼ�������ȶ��Ļ�����ʱ���ų�b kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽ________________________ ��
E������D����ȫȼ�������ȶ��Ļ�����ʱ���ų�b kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽ________________________ �� �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ������X����ˮ��ҺΪdz��ɫ���ɷ������µ�ת����ϵ�����ַ�Ӧ����������ԣ������У�B��D��E��F��Ϊ��ɫ���壬M��LΪ�����Ľ������ʣ�CΪ������ˮ�ĺ��ɫ���塣�ڻ��Һ�м���BaCl2��Һ�����ɲ�����ϡ����İ�ɫ������H��M��Ӧ�ɷų��������ȡ�
��ش��������⣺
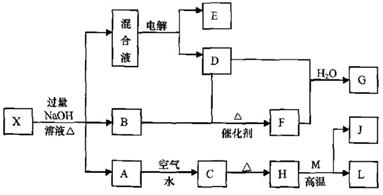 |
��1��B�ĵ���ʽΪ ��
��2������Ԫ��M��ԭ�ӽṹʾ��ͼ ��
��3������X�Ļ�ѧʽΪ ��
��4�������Һʱ������ӦʽΪ ��
��5����Ҫ��д������ת����ϵ���йط�Ӧ�Ļ�ѧ����ʽ��
�� ����LԪ�صĻ��Ϸ�Ӧ ��
�� ����LԪ�ص��û���Ӧ ��
��6����֪![]()
![]() E������D����ȫȼ�������ȶ��Ļ�����ʱ���ų�
E������D����ȫȼ�������ȶ��Ļ�����ʱ���ų�![]() kJ��������д��Eȼ���ȵ��Ȼ�ѧ����ʽ ��
kJ��������д��Eȼ���ȵ��Ȼ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com