

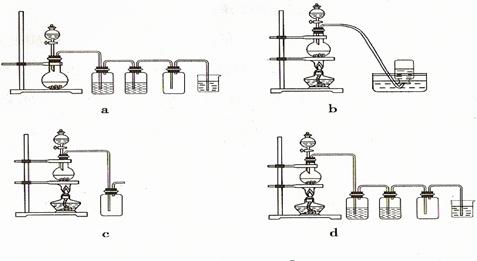
 Mn2����Cl2����2H2O��2�� ��
Mn2����Cl2����2H2O��2�� �� H++Cl��+HClO,����Cl����Ũ��ʹƽ�������ƶ����������ռ���������� ��2�� �� NaOH ��1�� �� Cl����2OH����Cl����ClO����H2O��2�� ��
H++Cl��+HClO,����Cl����Ũ��ʹƽ�������ƶ����������ռ���������� ��2�� �� NaOH ��1�� �� Cl����2OH����Cl����ClO����H2O��2�� ��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ó���ʯ��ˮ����Na2CO3��Һ��NaHCO3��Һ |
| B���õ�ȼ�ķ�����ȥCO2�е�CO |
| C���ü��ȷ���ȥNa2CO3��Һ�е�NaHCO3 |
| D����Ʒ����Һ������KMnO4��Һ���� CO2��SO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 CaCl2 + 2NH3��+ 2H2O
CaCl2 + 2NH3��+ 2H2O
 ��й©�¼���500 �������ҹ��ת�ơ����������ֳ���������ʲô�Ծ�Ϊ���� ��
��й©�¼���500 �������ҹ��ת�ơ����������ֳ���������ʲô�Ծ�Ϊ���� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A��AΪŨ���ᣬBΪŨ���ᣬ�Ƶ�HCl���� |
| B��AΪŨ��ˮ��BΪŨ����������Һ���Ƶ�NH3 |
| C��AΪŨ���ᣬBΪŨ���ᣬ�Ƶ�CH3COOH |
| D��AΪŨHI��Һ��BΪŨ���ᣬ�Ƶ�HI���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| | �۵� | �е� |
| PCl3 | -112�� | 75�� |
| PCl5 | 148�� | 200�� |
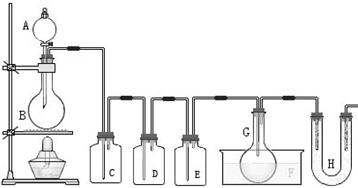
 ����F��ʢ�� ��������______________________��
����F��ʢ�� ��������______________________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ���ô��ű�ʾ)��
���ô��ű�ʾ)��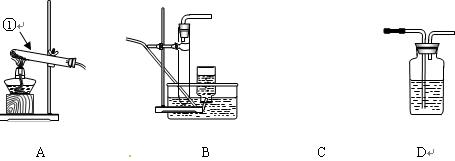
 ���ƶ�����̼ʱ��Ӧѡ��
���ƶ�����̼ʱ��Ӧѡ�� �ķ���װ����__ __�����������̼�����ѡ��Dװ
�ķ���װ����__ __�����������̼�����ѡ��Dװ �ã���װ����ʢ�ŵ��Լ�һ����__ __��
�ã���װ����ʢ�ŵ��Լ�һ����__ __���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com