
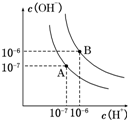
 ��֪ˮ��25���95��ʱ�������ƽ��������ͼ��ʾ��
��֪ˮ��25���95��ʱ�������ƽ��������ͼ��ʾ������ ��1��������������Ũ�ȣ�����������������Ũ�ȣ�ˮ�����ӻ�����Kw=c��H+����c��OH-�������A���ߵ�Kw��Ȼ����ˮ�ĵ�����������ж�25��ʱˮ�ĵ���ƽ�����ߣ�
��2��������Һ��pH�������Һ�������ӡ�����������Ũ�ȣ�����ʽ���������������Һ��������Һ�������
��3���ᡢ���ǿ����ʣ���Һ������˵�������Ӻ����������ӵ����ʵ�����ȣ����ˮ�����ӻ�����ȷ��ǿ���pHa��ǿ���pHb֮��Ӧ����Ĺ�ϵ��
��4�����¶���ˮ�����ӻ�Ϊ1��10-12���ݴ˼����pH=11��pH=2��pH=2����Һ�����������ӡ�������Ũ�ȣ�Ȼ����ʽ���㼴�ɣ�
��5����������B��Ӧ�¶���pH=5��˵����Һ��ʾ���ԣ���Ӧ�������ӹ���������
��� �⣺��1������A������Kw=c��H+����c��OH-��=10-7��10-7=10-14������B������c��H+��=c��OH-��=10-6 mol/L��Kw=c��H+��•c��OH-��=10-12��ˮ�ĵ���ʱ���ȹ��̣����ȴٽ����룬����A���ߴ���25��ʱˮ�ĵ���ƽ�����ߣ�
�ʴ�Ϊ��A��ˮ�ĵ��������ȹ��̣��¶ȵ�ʱ��ˮ�ĵ���̶�С��c��H+����c��OH-��С��
��2��25��ʱ���û����Һ��pH=7����Һ�����Լ����ǡ���кͣ���n��OH-��=n��H+������V��NaOH��•10-5 mol•L-1=V��H2SO4��•10-4 mol•L-1����V��NaOH����V��H2SO4��=10��1��
�ʴ�Ϊ��10��1��
��3��95��Cʱ��ˮ�����ӻ�Ϊ10-12�������Һ�����ԣ�˵�����������ӵ����ʵ������ڼ������������ӵ����ʵ���������10-amol/L��100=10b-12 mol/L��1������a+b=14��
�ʴ�Ϊ��pHa+pHb=14��
��4�����¶���ˮ�����ӻ�ΪKw=1��10-12����pH=11�Ŀ�����������������Ũ��Ϊ��c��OH-��=$\frac{1{0}^{-12}}{1{0}^{-11}}$mol/L=0.1mol/L��pH=1��ϡ������������Ũ��Ϊ0.1mol/L��
pH=2����Һ��������Ũ��Ϊ0.01mol/L������Һ�����㣺0.1mol/L��V2-0.1mol/L��V1=0.01mol/L����V1+V2����
�����ɵã�V1��V2=9��11��
�ʴ�Ϊ��9��11��
��5��������B��Ӧ�¶��£���pH���ᣩ+pH���=12���ɵ��������Һ��c��H+��=c��OH-��������ǿ������Һ�������Ϻ���Һ�����ԣ��ֻ����Һ��pH=5�����������Ϻ���Һ�����ԣ�˵��H+��OH-��ȫ��Ӧ�������µ�H+�������������������HA�����ᣬ
�ʴ�Ϊ������B��Ӧ95�棬��ʱˮ�����ӻ�Ϊ10-12��HAΪ���ᣬHA�к�NaOH�����Һ�л�ʣ��϶��HA���ӣ��ɼ��������H+��ʹ��ҺpH=5��
���� ���⿼����ˮ�ĵ��롢ˮ�����ӻ�����ҺpH�ļ��㣬��Ŀ�Ѷ��еȣ�����ؼ����ڸ�����¶ȶ�ˮ����ƽ�⡢ˮ�����ӻ�����ҺpH��Ӱ�죮


 ������ϵ�д�
������ϵ�д� �±�Сѧ��Ԫ�Բ���ϵ�д�
�±�Сѧ��Ԫ�Բ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
���鿴�𰸺ͽ���>>
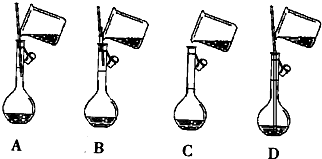
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ������ǰ��ϡ������ϴ����ƿ��δ������ˮϴ�� | |
| B�� | ����ҡ�Ⱥ���Һ����ڿ̶��ߣ��ټ�������ˮ����̶������� | |
| C�� | ϴ����Ͳ������ϴ��Һת������ƿ | |
| D�� | ����ʱ���Ӷ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
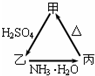 �ס��ҡ�����������֮��������ͼ��ʾ��ת����ϵ��
�ס��ҡ�����������֮��������ͼ��ʾ��ת����ϵ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
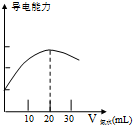 ijѧϰС����DISϵͳ�ⶨʳ�ð״��д�������ʵ���Ũ�ȣ�����Һ�ĵ����������жϵζ��յ㣮ʵ�鲽�����£�
ijѧϰС����DISϵͳ�ⶨʳ�ð״��д�������ʵ���Ũ�ȣ�����Һ�ĵ����������жϵζ��յ㣮ʵ�鲽�����£��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | K+��Na+��Cl-��CO32- | B�� | NH4+��Na+��Cl-��SO42- | ||
| C�� | Na+��HCO3-��NO3-��SO42- | D�� | K+��MnO4-��Na+��Cl- |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com