
T��ʱ����1 L���ܱ������г���2 mol CO2��6mol H2��һ�������·�����Ӧ��
CO2(g)+3H2(g) ![]() CH3OH(g)+H2O(g) ��H=��49.0kJ/mol
CH3OH(g)+H2O(g) ��H=��49.0kJ/mol
���H2��CH3OH(g)��Ũ����ʱ��仯����ͼ��ʾ������˵������ȷ���� �� ��

A��0~10min��v(H2)=0.3mol/(L��min)
B��T��ʱ��ƽ�ⳣ��![]() ��CO2��H2��ת�������
��CO2��H2��ת�������
C��T��ʱ��������Ӧ����64g CH3OH���ɣ�ͬʱ�ų�98.0kJ������
D���ﵽƽ��������¶Ȼ��ٳ���CO2���壬���������H2��ת����
 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ�����д�����ѧ��һ��ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ���ѡ��
T��ʱ����1 L���ܱ������г���2 mol CO2��6mol H2��һ�������·�����Ӧ��
CO2(g)+3H2(g)  CH3OH(g)+H2O(g) ��H=��49.0kJ/mol
CH3OH(g)+H2O(g) ��H=��49.0kJ/mol
���H2��CH3OH(g)��Ũ����ʱ��仯����ͼ��ʾ������˵������ȷ���� �� ��
| A��0~10min��v(H2)=0.3mol/(L��min) |
B��T��ʱ��ƽ�ⳣ��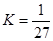 ��CO2��H2��ת������� ��CO2��H2��ת������� |
| C��T��ʱ��������Ӧ����64g CH3OH���ɣ�ͬʱ�ų�98.0kJ������ |
| D���ﵽƽ��������¶Ȼ��ٳ���CO2���壬���������H2��ת���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�걱���ж�����ʾ��У����12�½�ѧ�������л�ѧ�Ծ��������棩 ���ͣ�ѡ����
T��ʱ����1 L���ܱ������г���2 mol CO2��6 mol H2��һ�������·�����Ӧ��
CO2��g��+3H2��g�� CH3OH��g��+H2O��g�� ��H=-49.0 kJ/mol
CH3OH��g��+H2O��g�� ��H=-49.0 kJ/mol
���H2��CH3OH��g����Ũ����ʱ��仯�������ͼ��ʾ������˵������ȷ����
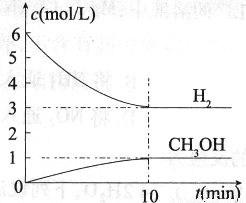
A��0��10 min��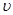 ��H2��=0.3 mol/��L��min��
��H2��=0.3 mol/��L��min��
B��T��ʱ��ƽ�ⳣ��K=1/27��CO2��H2��ת�������
C��T��ʱ����Ӧ�е���32 g CH3OH����ʱ���ų�49.0 kJ������
D���ﵽƽ��������¶Ȼ��ٳ���CO2���壬���������H2��ת����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�콭��ʡ�����и�һ��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
T��ʱ����1 L���ܱ������г���2 mol CO2��6mol H2��һ�������·�����Ӧ��
CO2(g)+3H2(g)  CH3OH(g)+H2O(g) ��H=��49.0kJ/mol
CH3OH(g)+H2O(g) ��H=��49.0kJ/mol
���H2��CH3OH(g)��Ũ����ʱ��仯����ͼ��ʾ������˵������ȷ���� �� ��
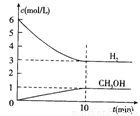
A��0~10min��v(H2)=0.3mol/(L��min)
B��T��ʱ��ƽ�ⳣ��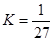 ��CO2��H2��ת�������
��CO2��H2��ת�������
C��T��ʱ��������Ӧ����64g CH3OH���ɣ�ͬʱ�ų�98.0kJ������
D���ﵽƽ��������¶Ȼ��ٳ���CO2���壬���������H2��ת����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��ʡ������ѧ�ڼ���ѧϰЧ����⿼�Ի�ѧ�Ծ� ���ͣ�ѡ����
T ��ʱ����1 L���ܱ������г���2 mol CO2��6 mol H2��һ�������·�����Ӧ��CO2(g)��3H2(g) 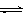 CH3OH(g)��H2O(g) ��H����49.0 kJ��mol-1���H2��CH3OH(g)��Ũ����ʱ��仯����ͼ��ʾ������˵������ȷ����:
CH3OH(g)��H2O(g) ��H����49.0 kJ��mol-1���H2��CH3OH(g)��Ũ����ʱ��仯����ͼ��ʾ������˵������ȷ����:
|
ʱ�� |
c ��H2����mol��L-1 |
c ��CH3OH����mol��L-1 |
v��������v ���棩�Ƚ� |
|
t0 |
6 |
0 |
�� |
|
t1 |
3 |
1 |
v������=v���棩 |
A��t0 ��t1ʱ������(H2)��3 /��t1 - t0�� mol��(L��min)-1
B�� t1ʱ���������¶Ȼ��ٳ���CO2���壬���������H2��ת����
C��t0ʱ��v��������v���棩
D��T ��ʱ��ƽ�ⳣ��K = 1/27��CO2��H2��ת�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�걱���г����������ڶ���ģ�⿼�ԣ����ۣ���ѧ���� ���ͣ�ѡ����
T��ʱ����1 L���ܱ������г���2 mol CO2��6mol H2��һ�������·�����Ӧ��
CO2(g)+3H2(g)  CH3OH(g)+H2O(g)
��H=��49.0kJ/mol
CH3OH(g)+H2O(g)
��H=��49.0kJ/mol
���H2��CH3OH(g)��Ũ����ʱ��仯����ͼ��ʾ������˵������ȷ���� �� ��
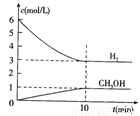
A��0~10min��v(H2)=0.3mol/(L��min)
B��T��ʱ��ƽ�ⳣ��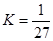 ��CO2��H2��ת�������
��CO2��H2��ת�������
C��T��ʱ��������Ӧ����64g CH3OH���ɣ�ͬʱ�ų�98.0kJ������
D���ﵽƽ��������¶Ȼ��ٳ���CO2���壬���������H2��ת����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com