
 HCO3-��OH��
HCO3-��OH�� HCO3-��OH����ʵ����з�Ӧ�����ӷ���ʽΪ3Fe2����NO3-��4H��=3Fe3����NO����2H2O��
HCO3-��OH����ʵ����з�Ӧ�����ӷ���ʽΪ3Fe2����NO3-��4H��=3Fe3����NO����2H2O��

 �����Ļ�������ҵϵ�д�
�����Ļ�������ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
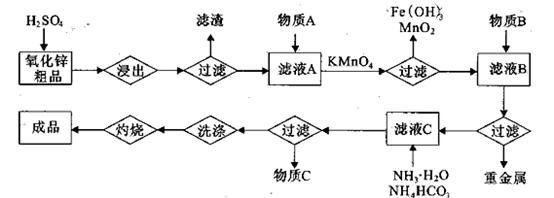
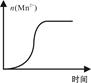
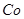 ����Mn2����
����Mn2����| A��NH3.H2O | B��Na2CO3 | C��H2SO4 | D��ZnO |
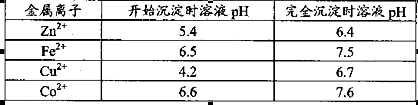
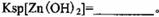 ��
��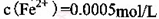 ������1
������1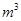 ����Һ��Fe2�������ĵ�KMnO4������Ϊ________g��������λ��Ч���֣���
����Һ��Fe2�������ĵ�KMnO4������Ϊ________g��������λ��Ч���֣���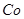 2���������û���Ӧ��ȥ��������B��_________��
2���������û���Ӧ��ȥ��������B��_________��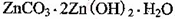 �����ɸó����Ļ�ѧ����ʽΪ________��
�����ɸó����Ļ�ѧ����ʽΪ________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| KMnO4������ҺŨ�� ��mol��L-1�� | ��Һ��ɫ����ʱ�䣨min�� | | ||
| ��һ�� | �ڶ��� | ������ | ||
| 0.02 | 14 | 13 | 11 | |
| 0.002 | 6.7 | 6.6 | 6.7 | |
| KMnO4������Һ | H2C2O4��Һ | ||
| Ũ��/ mol/L | ���(ml) | Ũ��/ mol/L | �����ml�� |
| 0.02 | 2 | b | 4 |
| a | 2 | c | 4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������ʣ�࣬��Һ��dz��ɫ��Cl��Ũ�Ȼ������� |
| B������Һ�е�����ɫ��KSCN��Һ����Ѫ��ɫ |
| C��Fe2����Fe3�������ʵ���֮��Ϊ5��1 |
| D�����������뻹ԭ��������ʵ���֮��Ϊ2��5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��NH3��ˮ��Һ���Ե��磬˵��NH3�ǵ���� |
| B����״���£�6.72 L NO2������ˮ��ȫ��Ӧת�Ƶ�����Ϊ1.204��1023 |
| C����ˮ��ͨ������������Ӧ�����ӷ���ʽΪ��Cl2��H2O=2H����Cl����ClO�� |
| D��������Ƭ�ܷų�H2����Һ�п��ܴ�����������ӣ�Na����NO3-��NH4+��SO42- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Fe3����Al3����Cl����NO3- | B��K����Na����I����SO42- |
| C��Ag����Ca2����NH4+��NO3- | D��Na����Ba2����CO32-��SO42- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������Һ�м���KI��Һ��ԭ�е������������ʵ������� |
| B�������Һ�еμ�ϡ����������Һ���������ְ�ɫ���� |
| C������Һ��c(Cl��)��0.6 mol��L��1�������Һ��pHΪ1 |
| D�������Һ�м���������ۣ�ֻ�����û���Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ������ | H����K����Al3����NH4+��Mg2�� |
| ������ | Cl����Br����OH����CO32-��AlO2- |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ����ѡ��Ba(OH)2��HCl��K2CO3�����Լ��������²��������
����ѡ��Ba(OH)2��HCl��K2CO3�����Լ��������²�������� 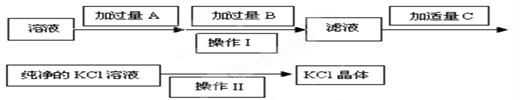
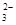 ��OH����HCO
��OH����HCO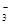 ��Cl���������е������֡�Ϊ��ȷ����Һ����ɣ����������²�����
��Cl���������е������֡�Ϊ��ȷ����Һ����ɣ����������²������鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com