
��8�֣����о���ʹ�õĹܵ�����ȼ���У�����Ȼ������Ҫ�ɷ���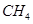 ���͡�ú��������Ҫ�ɷ���
���͡�ú��������Ҫ�ɷ���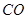 ��
��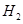 ��������ȼ�շ�Ӧ�Ļ�ѧ����ʽ���£�
��������ȼ�շ�Ӧ�Ļ�ѧ����ʽ���£�
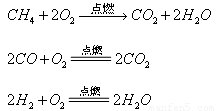
���жϣ�
��1��ȼ����ͬ����Ĺܵ�ú������Ȼ����������������ϴ�������֣�____________��
��2�������ڹܵ�ú��������ڸ�Ϊ������Ȼ��ʱ���轫����ڸĴ��Ǹ�С���粻�������ڣ����ܲ���ʲô���������_____________________________________��
��3���ܵ�ú���г�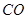 ��
��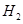 �⣬�����������顢���顢���顢���飨�е㣺-0.5�棩����̬���������Ϊʲô���Ϻ��Ķ������ܵ�ú����ʱ����ֻ����С���������
�⣬�����������顢���顢���顢���飨�е㣺-0.5�棩����̬���������Ϊʲô���Ϻ��Ķ������ܵ�ú����ʱ����ֻ����С���������
___________________________________________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� | H2 | CO | CH4 |
| ȼ���ȣ�kJ?mol-1�� | 285.8 | 283.0 | 890.3 |
| 1 |
| 2 |
| 1 |
| 2 |
| 1 |
| 2 |
| 1 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ������ʡ�̽��еڶ�����ѧ�߶�9���¿���ѧ�Ծ����������� ���ͣ������
��13�֣����ڳ��о���ʹ�õĹܵ�ú������Ҫ�ɷ���H2��CO������CH4��H2��CO��CH4��ȼ�����������±�
| ���� | H2 | CO | CH4 |
| ȼ���ȣ�kJ?mol-1�� | 285.8 | 283.0 | 890.3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014������ʡ�̽��и߶�9���¿���ѧ�Ծ��������棩 ���ͣ������
��13�֣����ڳ��о���ʹ�õĹܵ�ú������Ҫ�ɷ���H2��CO������CH4��H2��CO��CH4��ȼ�����������±�
|
���� |
H2 |
CO |
CH4 |
|
ȼ���ȣ�kJ•mol-1�� |
285.8 |
283.0 |
890.3 |
��1����д��H2��CO��CH4ȼ�յ��Ȼ�ѧ����ʽ��
_________________________________________________��
_________________________________________________��
_________________________________________________��
��2����Ϊ�����������������乤�̵���Ҫ�ɾͣ�������Ȼ����ȫ������Ϻ������վ��ڣ�½����Ϊ���о���ʹ�õ���Ҫ��Դ��ʹ�ùܵ�ú���û�������Ȼ����Ӧ������߽��������ţ�������_____�������������Ȼ�������Ľ����������_____�������������Ȼ�������Ľ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
| ���� | H2 | CO | CH4 |
| ȼ���ȣ�kJ?mol-1�� | 285.8 | 283.0 | 890.3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ������ʡ�̽����и߶����ϣ��¿���ѧ�Ծ���9�·ݣ��������棩 ���ͣ������
| ���� | H2 | CO | CH4 |
| ȼ���� | 285.8 | 283.0 | 890.3 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com