
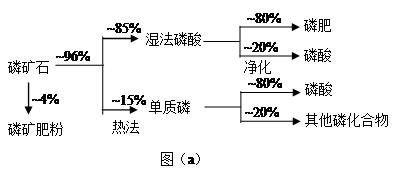
| | �۵�/�� | �е�/�� | ��ע |
| ���� | 44 | 280.5 | |
| PH3 | -133.8 | -87.8 | ������ˮ�����л�ԭ�� |
| SiF4 | -90 | -86 | ��ˮ�� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
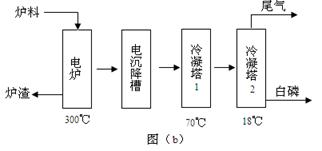
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������AgNO3��Һ���д�����ɫ�������� | B�������ᣬ����ɫ��ζ������� |
C������BaCl2��Һʱ�а�ɫ�����������ټ�ϡ��������ܽ⣬������ʹ����ʯ��ˮ����ǵ���ɫ��ζ������        | |
| D������Ba(OH)2��Һ�а�ɫ�����������ټ�ϡ������C���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��SO2ʹ����KMnO4��Һ��ɫ�� |
| B��Na2O2Ͷ���̪��Һ����Һ�ȱ�����ɫ |
| C����FeSO4��Һ�е�NaOH��Һ�����ɵij����ɰ�ɫ��ɻ���ɫ������ɺ��ɫ |
| D������ɫʯ����Һ��ͨ�백������Һ���� |
�鿴�𰸺ͽ���>>
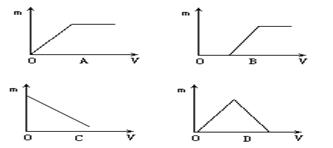
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��1�U4 | B��1�U5 | C��2�U3 | D��2�U5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ʵ������ȡ������Ϊ�˼ӿ췴Ӧ���ʣ�����ϡH2SO4�еμ�����Cu(NO3)2��Һ |
| B��Ϊ������¯ˮ���е�CaSO4�������ñ���Na2CO3��Һ���ݣ��ټ��������ܽ� |
C����֪NaOH(aq)��HCl(aq)��NaCl(aq)��H2O(l) ��H��-57.3 kJ��mol��1����ˮ������Ȼ�ѧ����ʽΪ��H2O(l) H+(aq)+OH-(aq)��H�� +57.3 kJ��mol��1 H+(aq)+OH-(aq)��H�� +57.3 kJ��mol��1 |
| D����������������ʴ�����ⸯʴ�ĸ�����Ӧ��Ϊ��Fe-2e- ��Fe2+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
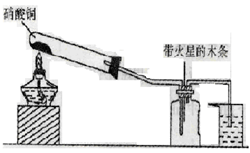
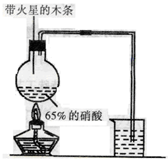
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
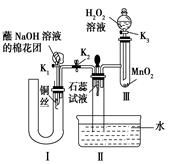
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com