
| ���� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0�� |
| 2 | �� | �� | �� | |||||
| 3 | �� | �� | �� | �� | �� | |||
| 4 | �� | �� |
 ��Ԫ�آ�����Ϊ�嵥����ˮ��Ӧ��ѧ����ʽBr2+H2O=HBr+HBrO��
��Ԫ�آ�����Ϊ�嵥����ˮ��Ӧ��ѧ����ʽBr2+H2O=HBr+HBrO�� ��
�� ���û������������ӣ�����ۡ������ӡ��������
���û������������ӣ�����ۡ������ӡ��������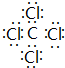 ��
�� ��
������ ��Ԫ�������ڱ��е�λ�ÿ�֪����ΪC����ΪN����ΪO����ΪNa����ΪSi����ΪS����ΪCl����ΪAr����ΪK����ΪBr��
��1��ϡ������Arԭ�������Ϊ�ȶ��ṹ����ѧ��������ã�ԭ�Ӻ�����18�����ӣ���3�����Ӳ㣬���������Ϊ2��8��8����ΪBr���嵥����ˮ��Ӧ����HBr��HBrO��
��2��������������ˮ�����У�������ǿ���Ǹ��������Ԫ����K�Ľ�������ǿ����KOH�ļ�����ǿ���ɼ����������������ӹ��ɣ�
��3���ڵ����������Ϊ���������������Ӧˮ����ΪHNO3��������Fe��ϡ���ᷴӦ��������������NO��ˮ��
��4��Ԫ�آ���Ļ�����ΪNa2S�����������������ӹ��ɣ�
��5��������γɵĻ�����ΪCCl4�����ڹ��ۻ����������̼ԭ������ԭ��֮���γ�1�Թ��õ��Ӷԣ�������γɵĻ�����ΪCS2�����ڹ��ۻ����������̼ԭ������ԭ��֮���γ�2�Թ��õ��Ӷԣ�
��6���ۡ����⻯��ֱ�ΪH2O��H2S������ˮ����֮�����������е�ϸߣ�
��7�����Ӳ�ṹ��ͬ�����ӣ��˵����Խ�����Ӱ뾶ԽС�����Ӳ�Խ�����Ӱ뾶Խ��
��8�����Ԫ��������������N��Sԭ����Ŀ֮�ȣ��ڽ����Է�������ȷ������ʽ��
��9���ܺ͢�����Ԫ���γɺ��зǼ��Լ��Ļ�����Ϊ�������ƣ�����û�������NaԪ�س��÷��� Ϊ��ɫ��Ӧ�����������������̼��Ӧ���Եõ�������ͬʱ����̼���ƣ�
��� �⣺��Ԫ�������ڱ��е�λ�ÿ�֪����ΪC����ΪN����ΪO����ΪNa����ΪSi����ΪS����ΪCl����ΪAr����ΪK����ΪBr��
��1��ϡ������Arԭ�������Ϊ�ȶ��ṹ����ѧ��������ã�ԭ�Ӻ�����18�����ӣ�ԭ�ӽṹʾ��ͼΪ ����Ϊ��Ԫ�أ��嵥����ˮ��Ӧ����HBr��HBrO����Ӧ����ʽΪ��Br2+H2O=HBr+HBrO��
����Ϊ��Ԫ�أ��嵥����ˮ��Ӧ����HBr��HBrO����Ӧ����ʽΪ��Br2+H2O=HBr+HBrO��
�ʴ�Ϊ��Ar�� ���壻Br2+H2O=HBr+HBrO��
���壻Br2+H2O=HBr+HBrO��
��2��������������ˮ�����У�������ǿ����HClO4������Ԫ����K�Ľ�������ǿ����KOH�ļ�����ǿ���ɼ����������������ӹ��ɣ�����ʽΪ�� ��
��
�ʴ�Ϊ��HClO4�� ��
��
��3���ڵ����������ΪN2O5�����Ӧˮ����ΪHNO3��������Fe��ϡ���ᷴӦ��������������NO��ˮ����Ӧ���ӷ���ʽΪ��3Fe+8H++2NO3-=3Fe2++2NO��+4H2O��
�ʴ�Ϊ��N2O5��3Fe+8H++2NO3-=3Fe2++2NO��+4H2O��
��4��Ԫ�آ���Ļ�����ΪNa2S�����������������ӹ��ɣ��������ӻ�����õ���ʽ��ʾ���γ�Ϊ�� ��
��
�ʴ�Ϊ�� �����ӣ�
�����ӣ�
��5��������γɵĻ�����ΪCCl4�����ڹ��ۻ����������̼ԭ������ԭ��֮���γ�1�Թ��õ��Ӷԣ�����ʽΪ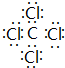 ��������γɵĻ�����ΪCS2�����ڹ��ۻ����������̼ԭ������ԭ��֮���γ�2�Թ��õ��Ӷԣ�����ʽΪ
��������γɵĻ�����ΪCS2�����ڹ��ۻ����������̼ԭ������ԭ��֮���γ�2�Թ��õ��Ӷԣ�����ʽΪ ��
��
�ʴ�Ϊ��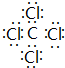 ��
�� ��
��
��6���ۡ����⻯��ֱ�ΪH2O��H2S������H2O����֮����������H2O�ķе����H2S���ʴ�Ϊ��H2O�ķе����H2S�� ����H2O����֮����������
��7�����Ӳ�ṹ��ͬ�����ӣ��˵����Խ�����Ӱ뾶ԽС�����Ӳ�Խ�����Ӱ뾶Խ�����Ӱ뾶��S2-��Cl-��Na+���ʴ�Ϊ��S2-��Cl-��Na+��
��8��S��N�γɵ�һ�ֻ�������Է���������170��190֮�䣬��S����������ԼΪ70%����NԪ����������Ϊ30%���������S��Nԭ����Ŀ֮��=$\frac{70%}{32}$��$\frac{30%}{14}$��1��1�������ʽΪ��SN��n����170��46n��190������ȷ��3.7��n��4.13����n=4�������ʽΪ��S4N4��
�ʴ�Ϊ��S4N4��
��9���ܺ͢�����Ԫ���γɺ��зǼ��Լ��Ļ�����ΪNa2O2������û�������NaԪ�س��÷���Ϊ��ɫ��Ӧ�����øû�������ȡ�����Ļ�ѧ����ʽΪ2Na2O2+2CO2=2Na2CO3+O2��
�ʴ�Ϊ��Na2O2����ɫ��Ӧ��2Na2O2+2CO2=2Na2CO3+O2��
���� ���⿼��Ԫ�������ɺ�Ԫ�����ڱ����ۺ�Ӧ�ã����س��û�ѧ����Ŀ��飬�ѶȲ���ע�����֪ʶ���������գ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���� | B�� | ��ȩ | C�� | ���� | D�� | �������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��״���£�2.24LSO3���еķ���������0.1NA | |
| B�� | 1 mol Na2O2������������Ϊ2NA | |
| C�� | ���³�ѹ�£�16gO2 ��O3�Ļ�����庬�е���ԭ����ΪNA | |
| D�� | ���³�ѹ���������£�33.6 LCl2��3.0g H2��Ӧ�����ɵ�HCl������ĿΪ3NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �߷���Ĥ | B�� | ҽ�ø߷��Ӳ��� | C�� | ����߷��Ӳ��� | D�� | Һ���߷��Ӳ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CH3OH | B�� | ��CH3��2CHOH | C�� | ��CH3��3COH | D�� | ��CH3��3CCH2OH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | A��B��C��D | B�� | D��A��B=C | C�� | A=B��C=D | D�� | D��A��B��C |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com