
(1)������FeS���ܽ��Ϊ_____________g������ʵ����Ũ��Զ���������ֵ����ԭ�������_________________________________________________________________��
(2)��֪FeS������Һ��c(H+)��c(S2-)֮���������������ϵ��c2(H+)��c(S2-)=1.0��10-22��Ϊ��ʹ��Һ��c(Fe2+)�ﵽ1 mol��L-1���ֽ�����FeSͶ���䱥����Һ�У�Ӧ������Һ�е�c(H+)Ϊ_____________mol��L-1��
������(1)FeS(s)![]() Fe2++S2-����ˮ�����ӻ����ƣ��������ܽ��FeS�����������������Ũ�ȳ˻�ҲΪ���������ܶȻ���������Ksp��ʾ��Ksp����Ũ��Ӱ�죬һ��ֻ���¶�Ӱ�졣��FeS������Һ�У�c(Fe2+)=c(S2-)=
Fe2++S2-����ˮ�����ӻ����ƣ��������ܽ��FeS�����������������Ũ�ȳ˻�ҲΪ���������ܶȻ���������Ksp��ʾ��Ksp����Ũ��Ӱ�죬һ��ֻ���¶�Ӱ�졣��FeS������Һ�У�c(Fe2+)=c(S2-)=![]() =9��10-9 mol��L-1�����䱥����ҺΪ1 L(����FeS�ܽ�Ⱥ�С������Һ���ܶȽ���Ϊ
=9��10-9 mol��L-1�����䱥����ҺΪ1 L(����FeS�ܽ�Ⱥ�С������Һ���ܶȽ���Ϊ![]() HS-+OH-��Fe2++2H2O
HS-+OH-��Fe2++2H2O![]() Fe(OH)2+2H+�����������Ӿ�����Һ�з���ˮ�⣬ʹc(Fe2+)��c(S2-)���ͣ���ʹFeS(s)
Fe(OH)2+2H+�����������Ӿ�����Һ�з���ˮ�⣬ʹc(Fe2+)��c(S2-)���ͣ���ʹFeS(s) ![]() Fe2++S2-���ܽ�ƽ�����ܽ�ķ�����С�
Fe2++S2-���ܽ�ƽ�����ܽ�ķ�����С�
(2)����Һ��c(Fe2+)=1mol��L-1ʱ��c(S2-)=![]() =8.1��10-17mol��L-1������c2(H+)��c(S2-)=1��10-22������Һ��c2(H+)=
=8.1��10-17mol��L-1������c2(H+)��c(S2-)=1��10-22������Һ��c2(H+)=![]() ��c(H+)��1.11��10-3 mol��L-1 ��
��c(H+)��1.11��10-3 mol��L-1 ��
�𰸣�(1)7.92��10-8��S2-+H2O![]() HS-+OH-��Fe2++2H2O
HS-+OH-��Fe2++2H2O![]() Fe(OH)2+2H+�����������Ӿ�����Һ�з���ˮ�⣬ʹc(Fe2+)��c(S2-)���ͣ���ʹFeS(s)
Fe(OH)2+2H+�����������Ӿ�����Һ�з���ˮ�⣬ʹc(Fe2+)��c(S2-)���ͣ���ʹFeS(s) ![]() Fe2++S2-���ܽ�ƽ�����ܽ�ķ�����С�
Fe2++S2-���ܽ�ƽ�����ܽ�ķ�����С�
(2)1.11��10-3 mol��L-1



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ��ѧ�� | C-C | C-H | C-O | Si-Si | Si-H | Si-O |
| ����/��kJ?mol-1�� | 356 | 413 | 336 | 226 | 318 | 452 |
4- 4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
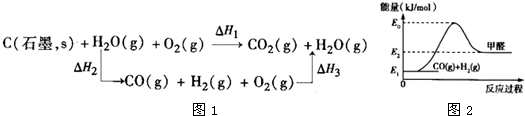
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�л����ڷ�Ӧ�г���ԭ���ʵĹ��ۼ�ȫ���ֶ��ѣ���C-H��C-C��C=C��C-0��C=O�ȣ����и���Ӧ�У���Ӧ������ж��Ѽ�����ȷ����ǣ�������
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 W��ij������Ԫ��X�Ĺ�̬���ʣ�A��B��C��Ϊ��XԪ�ص���ʽ���Σ�������һ������������ͼ��ʾ��ת����ϵ������ijЩ��Ӧ�����е�
W��ij������Ԫ��X�Ĺ�̬���ʣ�A��B��C��Ϊ��XԪ�ص���ʽ���Σ�������һ������������ͼ��ʾ��ת����ϵ������ijЩ��Ӧ�����е�

| 7 |
| 4 |
| 7 |
| 4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ȫ����ͨ�ߵ�ѧУ����ͳһ���������ۺ��������Ի�ѧ���¿α��������棩 ���ͣ������
[��ѧ��ѡ��3�����ʽṹ������]��15�֣�
������Ҫ�İ뵼����ϣ��������ִ����ӹ�ҵ�Ļ�������ش��������⣺
��1����̬Siԭ���У�����ռ�ݵ�����ܲ����Ϊ �����ܲ���е�ԭ�ӹ����Ϊ ��������Ϊ ��
��2������Ҫ�Թ����Ρ� �Ȼ��������ʽ�����ڵؿ��С�
��3�����ʹ��������ʯ�ṹ���Ƶľ��壬����ԭ����ԭ��֮���� ���ϣ��侧���й���8��ԭ�ӣ�����������λ�ù��� ��ԭ�ӡ�
��4�����ʹ��ͨ�����飨SiH4���ֽⷴӦ���Ʊ�����ҵ�ϲ���Mg2Si��NH4Cl��Һ�������з�Ӧ�Ƶ�SiH4���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��5��̼����йػ�ѧ������������ʾ����Ҫ�����ͽ��������й���ʵ��
|
��ѧ�� |
C��C |
C��H |
C��O |
Si��Si |
Si��H |
Si��O |
|
����/(kJ?mol-1 |
356 |
413 |
336 |
226 |
318 |
452 |
�ٹ���̼ͬ�壬Ҳ��ϵ���⻯�������������������϶�Զ���������࣬ԭ���� ��
��SiH4���ȶ���С��CH4���������������ԭ���� ��
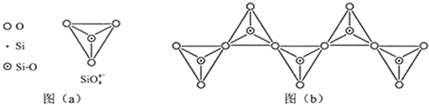
��6���ڹ������У�SiO4- 4�����壨����ͼ��a����ͨ�����ö��������ӿ��γɵ�״����״����״���Ǽ���״�Ĵ���ṹ��ʽ��ͼ��b��Ϊһ�����������ṹ�Ķ�����������Siԭ�ӵ��ӻ���ʽΪ ��Si��O��ԭ����֮��Ϊ ����ѧʽΪ ��

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com