
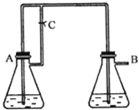
 Fe��OH��2�����ڿ������ױ���������ȡʱ���ѹ۲쵽��ɫ��������ֻ�ܿ�������ɫ��������ͼװ��ʹ��Fe��H2SO4��ϡ����NaOH��Һ���ڻ�ԭ����������ȡFe��OH��2��ɫ�������ҽϳ�ʱ���ڲ���ɫ������CΪ���ɼУ�����ʾ����ԭ������������������
Fe��OH��2�����ڿ������ױ���������ȡʱ���ѹ۲쵽��ɫ��������ֻ�ܿ�������ɫ��������ͼװ��ʹ��Fe��H2SO4��ϡ����NaOH��Һ���ڻ�ԭ����������ȡFe��OH��2��ɫ�������ҽϳ�ʱ���ڲ���ɫ������CΪ���ɼУ�����ʾ����ԭ������������������


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��14�֣�Fe��OH��2�����ڿ������ױ���������ȡʱ���ѹ۲쵽��ɫ��������ֻ�ܿ�������ɫ��������ͼװ��ʹ��Fe��H2SO4��ϡ����NaOH��Һ���ڻ�ԭ����������ȡFe��OH��2��ɫ�������ҽϳ�ʱ���ڲ���ɫ������CΪ���ɼС�����ʾ����ԭ������������������
��1������ƿA��Ӧ�����ҩƷΪ_________________.��2�֣�
��2����ƿB��Ӧ�����ҩƷΪ__________________����2�֣�
��3������A�еķ�Ӧ��ʼ�����д������Ʊ�Fe��OH��2�ĺ����������衣
��_____________________ ��_______________________________����4�֣�
��4�����ڷ�Ӧ��ʼ֮ǰ�ȹرյ��ɼ�C����ʵ������Ϊ��
����2�֣�
��д����ʱBƿ�з����Ļ�ѧ��Ӧ�����ӷ���ʽ��
����4�֣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010����������һ�и�����ѧ�ڵڶ����¿������ۣ���ѧ���� ���ͣ�ʵ����
��14�֣�Fe��OH��2�����ڿ������ױ���������ȡʱ���ѹ۲쵽��ɫ��������ֻ�ܿ�������ɫ��������ͼװ��ʹ��Fe��H2SO4��ϡ����NaOH��Һ���ڻ�ԭ����������ȡFe��OH��2��ɫ�������ҽϳ�ʱ���ڲ���ɫ������CΪ���ɼС�����ʾ����ԭ������������������
��1������ƿA��Ӧ�����ҩƷΪ_________________.��2�֣�
��2����ƿB��Ӧ�����ҩƷΪ__________________����2�֣�
��3������A�еķ�Ӧ��ʼ�����д������Ʊ�Fe��OH��2�ĺ����������衣
��_____________________ ��_______________________________����4�֣�
��4�����ڷ�Ӧ��ʼ֮ǰ�ȹرյ��ɼ�C����ʵ������Ϊ��
����2�֣�
��д����ʱBƿ�з����Ļ�ѧ��Ӧ�����ӷ���ʽ��
����4�֣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�����ĸ�����ѧ�ڵڶ����¿������ۣ���ѧ���� ���ͣ�ʵ����
��14�֣�Fe��OH��2�����ڿ������ױ���������ȡʱ���ѹ۲쵽��ɫ��������ֻ�ܿ�������ɫ��������ͼװ��ʹ��Fe��H2SO4��ϡ����NaOH��Һ���ڻ�ԭ����������ȡFe��OH��2��ɫ�������ҽϳ�ʱ���ڲ���ɫ������CΪ���ɼС�����ʾ����ԭ������������������

��1������ƿA��Ӧ�����ҩƷΪ_________________.��2�֣�
��2����ƿB��Ӧ�����ҩƷΪ__________________����2�֣�
��3������A�еķ�Ӧ��ʼ�����д������Ʊ�Fe��OH��2�ĺ����������衣
��_____________________ ��_______________________________����4�֣�
��4�����ڷ�Ӧ��ʼ֮ǰ�ȹرյ��ɼ�C����ʵ������Ϊ��
����2�֣�
��д����ʱBƿ�з����Ļ�ѧ��Ӧ�����ӷ���ʽ��
����4�֣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Fe��OH��2�����ڿ������ױ���������ȡʱ���ѹ۲쵽��ɫ��������ֻ�ܿ�������ɫ��������ͼװ��ʹ��Fe��H2SO4��ϡ����NaOH��Һ���ڻ�ԭ����������ȡFe��OH��2��ɫ�������ҽϳ�ʱ���ڲ���ɫ������CΪ���ɼС�����ʾ����ԭ������������������

��1������ƿA��Ӧ�����ҩƷΪ_________________.
��2����ƿB��Ӧ�����ҩƷΪ__________________��
��3������A�еķ�Ӧ��ʼ�����д������Ʊ�Fe��OH��2�ĺ����������衣
��_____________________ ��_______________________________��
��4�����ڷ�Ӧ��ʼ֮ǰ�ȹرյ��ɼ�C����ʵ������Ϊ��
��
��д����ʱBƿ�з����Ļ�ѧ��Ӧ�����ӷ���ʽ��
��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com