
| �ζ����� | ������Һ�����/mL | ����Һ����� | |
| �ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
| 1 | 25.00 | 1.02 | 21.04 |
| 2 | 25.00 | 1.00 | 21.00 |
| 3 | 25.00 | 1.20 | 21.21 |
���� ��1����A����ȡ�ζ��յ�ʱNaOH��Һ�����ʱ���ӿ̶��ߣ���ȡ������������Һ�����ƫС��
B���ζ�ǰ�ζ��ܼ��������ݣ��ζ����������ݣ�������ȥ������������Һ�����ƫ��
C��ʢװ��Һ�ĵζ���װҺǰ������ˮ��ϴ����δ�ñ�Һ��ϴ�����±�ҺŨ��ƫС��������ȥ������������Һ�����ƫ��
D���μ�NaOH��Һ���죬δ������տ�����Һ��ɫ������ֹͣ�ζ���������ȥ������������Һ�����ƫС��
�ڵζ�ʱ�۾�Ӧע��ע��۲���ɫ�仯����ȷ���յ㣻
�۸��ݷ�̪�ı�ɫ��Χȷ���ζ��յ�ʱ��ɫ�仯��
��������Ʒ��Һ���ζ�ʵ��������IJ����������ձ���250mL����ƿ����ƿ����Ͳ���ζ��ܡ�����������ͷ�ιܣ�
��2������Һ�����Ӧȡ����ʵ���ƽ��ֵ���������Һ��H+�����ʵ��������ݷ���ʽ��֪��CH2��6N4H+�����ʵ���������ȷ����Ʒ�е�������������
��� �⣺��1����A����ȡ�ζ��յ�ʱNaOH��Һ�����ʱ���ӿ̶��ߣ���ȡ������������Һ�����ƫС���ᵼ�¼���õģ�NH4��2SO4��Ʒ�е�����������ƫ�ͣ�
B���ζ�ǰ�ζ��ܼ��������ݣ��ζ����������ݣ�������ȥ������������Һ�����ƫ�ᵼ�¼���õģ�NH4��2SO4��Ʒ�е�����������ƫ�ߣ�
C��ʢװ��Һ�ĵζ���װҺǰ������ˮ��ϴ����δ�ñ�Һ��ϴ�����±�ҺŨ��ƫС��������ȥ������������Һ�����ƫ�ᵼ�¼���õģ�NH4��2SO4��Ʒ�е�����������ƫ�ߣ�
D���μ�NaOH��Һ���죬δ������տ�����Һ��ɫ������ֹͣ�ζ���������ȥ������������Һ�����ƫС���ᵼ�¼���õģ�NH4��2SO4��Ʒ�е�����������ƫ�ͣ�
��ѡBC��
�ڶ�ʱ�ߵα�ҡ����ƿ���۾�Ӧע��۲���ɫ�仯��ȷ���ζ��յ㣬�ʴ�Ϊ��B��
�۴���ҺΪ���ԣ���̪ӦΪ��ɫ������ҺתΪ����ʱ����Һ��ɫ��Ϊ�ۺ죨��dz�죩�����һ��NaOH��Һ���£���Һ����ɫ���ۺ죨��dz�죩���Ұ�����ڲ���ɫ��
�ʴ�Ϊ����ɫ���ۺ죨��dz�죩��
��������Ʒ��Һ���ζ�ʵ��������IJ����������ձ���250mL����ƿ����ƿ����Ͳ���ζ��ܡ�����������ͷ�ιܣ�
�ʴ�Ϊ������������ͷ�ιܣ�
��2������Һ�����Ӧȡ����ʵ���ƽ��ֵ��
����ȷ���ζ�ʱ���õ�NaOH����ҺΪ$\frac{20.02+20.00+20.01}{3}$mL=20.01mL��
�����������Լ�ȩ��Һһ���ǹ����ģ�����1.500g ��� ���ܽ��ȡ������$\frac{1}{10}$���еζ�����0.15g��
�ζ��������Һ�к���H+������CH2��6N4H+����0.0201L��0.1000mol/L=0.00201mol��
����4NH4++6HCHO�T3H++6H2O+��CH2��6N4H+��ÿ����4molH+������CH2��6N4H+����������NH4+4mol��
���Թ�����NH4+0.00201mol��
���к���Ԫ��0.00201mol��14g/mol=0.02814g
���Ե�����������Ϊ$\frac{0.02814g}{0.15g}$��100%=18.76%��
�ʴ�Ϊ��18.76%��
���� ���⿼�����ʵĺ����IJⶨ���������к͵ζ��Ŀ��飬ע����ѧ��ʵ�������ͷ��������ͼ����������ۺϿ��飬Ϊ���Ը�Ƶ���㣬��Ŀ�ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ȼ�շ�Ӧ | B�� | ȡ����Ӧ | C�� | ������Ӧ | D�� | �ӳɷ�Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ʯ�͵Ĵ�������Ϊ�˻��������Ϊ���������� | |
| B�� | ��������Ҫ������ú������ú���� | |
| C�� | ���͡�ú�͡�������Ҫ������ʯ�͵ij�ѹ���� | |
| D�� | ��ϩ����ʯ���ѽ�õ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��ش��������⣺
��ش��������⣺| ��ѧ�� | H-H | O�TO | O-H |
| E/kJ•mol-1 | 436 | x | 463 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Mg2+��NH${\;}_{4}^{+}$��OH-��SO${\;}_{4}^{2-}$ | B�� | Na+��H+��Cl-��HCO${\;}_{3}^{-}$ | ||
| C�� | Na+��K+��AlO${\;}_{2}^{-}$��CO${\;}_{3}^{2-}$ | D�� | H++��K+��MnO${\;}_{4}^{-}$��SO${\;}_{3}^{2-}$ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1��ȡ������Ʒ����������������� Һ�ֳ����ݣ��ֱ�װ��A��B�Թ��У� | |
| ����2���ýྻ�IJ�˿պȡA�е���Һ���ھƾ��ƻ��������գ��۲���ɫ | ��ɫ�ʻ�ɫ֤����Na+�����ͷ�����NaHCO3 |
| ����3����B�Թ�����εμ�0.1mol/L NaOH��Һ | �����а�ɫ�������ɣ����Ȳ�����ɫ����������ܽ⣩��֤�����ͷ����������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
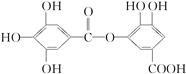 �������������������в���ȷ���ǣ�������
�������������������в���ȷ���ǣ�������| A�� | ���������¿ɷ���ˮ�ⷴӦ���Ҳ���ֻ��һ�� | |
| B�� | 1mol���л������ɸ�8molNaOH��Ӧ | |
| C�� | 1mol���л������ɸ�2molBr2��Ӧ | |
| D�� | ���л���ɸ�NaHCO3��Һ��Ӧ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com