
��1���������Ͽ���ѹǿ����ƽ��������Ӧ�����ƶ���SO2��ת����������ҵ�϶���������������ó�ѹ�����ø�ѹ����Ҫ��ԭ����____________________________��
��2�����������¶Ⱥ�ѹǿ���䣬����������ֻ����2 mol SO3������������������ƽ��ʱSO2�����������________����ʱƽ��������������________��
��3���¶��Ա���537 �棬�������������200 L���䡣����a mol SO2��b mol O2������������������Ӧ�ﵽƽ��ʱ��SO3�����������Ϊ0.91����ϵѹǿΪ1.01��105 Pa����a��b=2��1����a=________��
��1����ѹ�£�SO2��ת�����Ѵﵽ��94%������ѹǿ��ת������ߵ����ޣ����Զ������豸��Ҫ����ߣ������ھ���Ч������� ��2��6%137.5 L ��3��2.91
����������Ե�Чƽ����������Чƽ��ľ���Ӧ�á�
��ԭƽ���������O2ת�������ʵ���Ϊx����
2SO2 + O2![]() 2SO3
2SO3
��ʼ��mol�� 2 1 0
ת����mol�� 2x x 2 x
ƽ�⣨mol�� 2-2x 1-x 2x
SO3���������=![]() =0.91,x=0.938��
=0.91,x=0.938��
SO2���������=![]() =0.06
=0.06
V��ƽ�⣩=![]() ��200 L=
��200 L=![]() ��200 L=137.5 L
��200 L=137.5 L
��Ҳ��ͨ�������ϵ����ƽ��ʱSO2�������������ʼ�����SO2��O2������������ĵ��������ͬ����ʣ���SO2��O2������ٷֱ�ҲΪ2��1����SO3���������Ϊ0.91����ʣ���0.09�У�SO2��O2������ٷֱȷֱ�Ϊ0.06��0.03��
�����¶Ⱥ�ѹǿ���䣬��������ֻ����SO3�����ﵽ��ƽ����ԭƽ���Ч��SO2�����������ԭƽ��һ����������SO3Ϊ2 mol��������������2 mol SO2(g)��1 mol O2(g)��ƽ��ʱ�����ȡ�
�¶ȱ���537 �棬�������������200 L���䣬����ԭƽ���Ч����SO3�����ʵ���Ϊ![]() ��2 mol=2.91 mol��
��2 mol=2.91 mol��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 4V |
| 3 |
| 4V |
| 3 |
| 4V |
| 3 |
| 4V |
| 3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��537�桢1.01��105 Paʱ�����ݻ��ɱ���ܱ������г���1 mol X��3 mol Y����ʱ�ݻ�ΪV L�����ֺ��º�ѹ��������ӦX(g)+3Y(g)![]() 2Z(g)���ﵽƽ��ʱ��ƽ����������Z���������Ϊ0.5��
2Z(g)���ﵽƽ��ʱ��ƽ����������Z���������Ϊ0.5��
��1���ﵽƽ��ʱ��X��ת����ԼΪ ��
��2�����������¶Ⱥ�ѹǿ�㶨���䣬����������ֻ����4 mol Z����Ӧ�ﵽƽ��ʱ��ƽ��������Y ���������Ϊ ���������ݻ�Ϊ L��
��3������ѡһ�ݻ��̶�������ܱ��������Կ����¶Ȳ��䣬ʹ2 mol X��6 mol Y��Ӧ���ﵽƽ��ʱƽ��������Z�����������Ϊ0.5������ܱ��������ݻ�Ϊ ��
��4�����¶���Ϊ537�棬�����������VL����(����)�������г���a mol X��b mol Y��ʹ��Ӧ�ﵽƽ�⣬��ʱƽ��������Z�����������Ϊ0.5����ϵѹǿΪ1.01��105 Pa����a: b = 1: 3����a = ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��537�桢1.01��105 Paʱ�����ݻ��ɱ���ܱ������г���1 mol X��3 mol Y����ʱ�ݻ�ΪV L�����ֺ��º�ѹ��������ӦX(g)+3Y(g)![]() 2Z(g)���ﵽƽ��ʱ��ƽ����������Z���������Ϊ0.5��
2Z(g)���ﵽƽ��ʱ��ƽ����������Z���������Ϊ0.5��
��1���ﵽƽ��ʱ��X��ת����ԼΪ ��
��2�����������¶Ⱥ�ѹǿ�㶨���䣬����������ֻ����4 mol Z����Ӧ�ﵽƽ��ʱ��ƽ��������Y ���������Ϊ ���������ݻ�Ϊ L��
��3������ѡһ�ݻ��̶�������ܱ��������Կ����¶Ȳ��䣬ʹ2 molX��6 mol Y��Ӧ���ﵽƽ��ʱƽ��������Z�����������Ϊ0.5������ܱ��������ݻ�Ϊ ��
��4�����¶���Ϊ537�棬�����������VL����(����)�������г���a mol X��b mol Y��ʹ��Ӧ�ﵽƽ�⣬��ʱƽ��������Z�����������Ϊ0.5����ϵѹǿΪ1.01��105 Pa����a: b = 1: 3����a = ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ��Ҧ��ѧ�߶���ѧ�����п���ʵ��ѧ�Ծ����������� ���ͣ������
(10��)����537�桢1.01��105Paʱ�����ݻ��ɱ���ܱ������г���1molX��3molY����ʱ�ݻ�ΪVL�����ֺ��º�ѹ��������ӦX(g)+ 3Y(g) 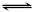 2Z(g)���ﵽƽ��ʱ��ƽ����������Z���������Ϊ0.5��
2Z(g)���ﵽƽ��ʱ��ƽ����������Z���������Ϊ0.5��
��1���ﵽƽ��ʱ��X��ת����ԼΪ ��
��2�����������¶Ⱥ�ѹǿ�㶨���䣬����������ֻ����4molZ����Ӧ�ﵽƽ��ʱ��ƽ��������Y���������Ϊ ���������ݻ�Ϊ L��
��3������ѡһ�ݻ��̶�������ܱ��������Կ����¶Ȳ��䣬ʹ2molX��6molY��Ӧ���ﵽƽ��ʱƽ��������Z�����������Ϊ0.5������ܱ��������ݻ�Ϊ
��4�����¶���Ϊ537�棬�����������VL���䣨���ݣ��������г���a mol X��b mol Y��ʹ��Ӧ�ﵽƽ�⣬��ʱƽ��������Z�����������Ϊ0.5����ϵѹǿΪ1.01��105Pa����a : b =" 1" : 3����a=
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�����������ѧ�߶���ѧ�����п������ƻ�ѧ�Ծ��������棩 ���ͣ������
��8�֣���537�桢101KPa ʱ�����ݻ��ɱ���ܱ������г���2mol SO2�� 1mol O2����ʱ���������Ϊ200L���������м�����������壩�����ֺ��º�ѹ��������Ӧ��2SO2(g)+O2(g)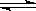 2SO3(g)�ﵽƽ��ʱ��ƽ��������SO3���������Ϊ91%���Իش��������⣺
2SO3(g)�ﵽƽ��ʱ��ƽ��������SO3���������Ϊ91%���Իش��������⣺
��1�����������¶Ⱥ�ѹǿ���䣬����������ֻ����2mol SO3����������������ƽ��ʱ��SO2����������� �����������Ϊ L��
��2�����¶Ⱥ��������ݻ����䣬��ƽ��ʱ�������������ѹǿΪ KPa��
��3���¶��Ա���537�棬�����������200L���䣨���ݣ�������a molSO2��b molO2������������������Ӧ��ƽ��ʱ��SO3�����������Ϊ0.91����ϵѹǿΪ101KPa����a:b=2:1����a= ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com