
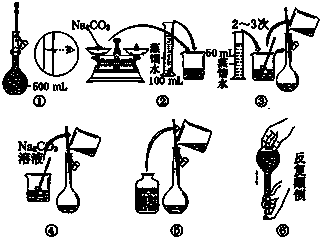
 ��1����ijҩƷ����ԼΪ32g
��1����ijҩƷ����ԼΪ32g| 50g | 20g | 20g | 10g | 5g |
| n |
| V |
 ��
��

 �����Ļ���������人������ϵ�д�
�����Ļ���������人������ϵ�д� ���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д�
���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
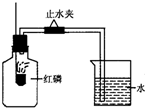 ��֪��������Ҫ�ɷ��ǵ�����������ij����С����Ʋⶨ����������������ʵ�飬ʵ��װ����ͼ��ʾ��
��֪��������Ҫ�ɷ��ǵ�����������ij����С����Ʋⶨ����������������ʵ�飬ʵ��װ����ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Һ̬�ƿ������˷�Ӧ�ѵĴ��Ƚ��� |
| B���ڹ���Ԫ������Ѱ�뵼����� |
| C������ʱ����l4C�ⶨһЩ�������� |
| D��SiO2������ˮ�ࡢ���챦ʯ���������ԭ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����NaHCO3�������������ˮ�У�����ɫ���ݣ�H+�� |
| B��ʹ��ɫ������ɫ��HCl�� |
| C����FeCl2��Һ�еμ���ˮ���ٵμ�KSCN��Һ�����ֳʺ�ɫ��Cl2�� |
| D���μ�AgNO3��Һ���ɰ�ɫ������Cl-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
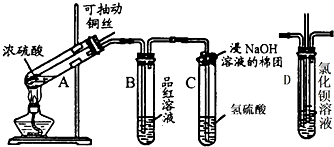
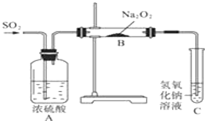 ������ʵ��С��̽��Na2O2��SO2�ķ�Ӧ��������ͼ��ʾװ�ý���ʵ�飮
������ʵ��С��̽��Na2O2��SO2�ķ�Ӧ��������ͼ��ʾװ�ý���ʵ�飮
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
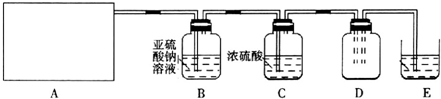
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1��ȡ����ϴ��ƿB����Һ���Թܢ��У��μ� | �������İ�ɫ��������ϴ��ƿB��Һ�д���SO42-�� |
| ����2����ȡ����ϴ��ƿB����Һ���Թܢ��У��μ� ����Ba��NO3��2��Һ�����ã� | ������ɫ������ |
| ����3��ȡ����2���Թܢ��е��ϲ���Һ���Թܢ��У��μ� | ��������ɫ��������ϴ��ƿB��Һ�д���Cl- |
| ʵ�鲽�� | ʵ����� | ||||
| �� | ȡ����������FeCO2�������������У������������������ټ��ᣬ��ȴ������ | ||||
| �� | ȡ����ʵ�鲽������ù������һ�ྻ���Թ��У���������ϡ�����ܽ� | ||||
| �� | ��ʵ�鲽���������Һ�еμ�KSCN��Һ����Һ��� | ||||
��ͬѧ�Ľ��ۣ�4FeCO3+O2
| |||||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ֻ�Т� | B��ֻ�Т٢� |
| C��ֻ�Т٢� | D��ȫ����ȷ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com