

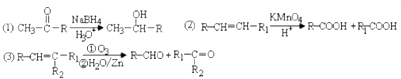
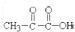 ��E�����ͬϵ����Ա�ͪ��Ϊԭ�Ϻϳ��Ҷ��ᣨ�úϳ�·������ͼ��ʾ����ע����Ӧ������
��E�����ͬϵ����Ա�ͪ��Ϊԭ�Ϻϳ��Ҷ��ᣨ�úϳ�·������ͼ��ʾ����ע����Ӧ������| Ũ���� |
| 170�� |
| Br2 |
| 2��7+2-10 |
| 2 |
 ��X������ԭ��Ӧ����AΪHOOC-CH=CH-CH2-CH2-CH��OH��-CH3��A�����������ӳɷ�Ӧ����BΪHOOC-��CH2��4-CH��OH��-CH3��B������ȥ��Ӧ����CΪHOOC-CH2-CH2-CH2-CH=CHCH3��C������Ϣ�г�����������������ȩ��D����DΪHOOC-CH2-CH2-CH2-CHO���ݴ˽��
��X������ԭ��Ӧ����AΪHOOC-CH=CH-CH2-CH2-CH��OH��-CH3��A�����������ӳɷ�Ӧ����BΪHOOC-��CH2��4-CH��OH��-CH3��B������ȥ��Ӧ����CΪHOOC-CH2-CH2-CH2-CH=CHCH3��C������Ϣ�г�����������������ȩ��D����DΪHOOC-CH2-CH2-CH2-CHO���ݴ˽��| 2��7+2-10 |
| 2 |
 ��X������ԭ��Ӧ����AΪHOOC-CH=CH-CH2-CH2-CH��OH��-CH3��A�����������ӳɷ�Ӧ����BΪHOOC-��CH2��4-CH��OH��-CH3��B������ȥ��Ӧ����CΪHOOC-CH2-CH2-CH2-CH=CHCH3��C������Ϣ�г�����������������ȩ��D����DΪHOOC-CH2-CH2-CH2-CHO��
��X������ԭ��Ӧ����AΪHOOC-CH=CH-CH2-CH2-CH��OH��-CH3��A�����������ӳɷ�Ӧ����BΪHOOC-��CH2��4-CH��OH��-CH3��B������ȥ��Ӧ����CΪHOOC-CH2-CH2-CH2-CH=CHCH3��C������Ϣ�г�����������������ȩ��D����DΪHOOC-CH2-CH2-CH2-CHO�� ��
�� ��
��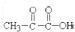 ��E�����ͬϵ��Ա�ͪ��Ϊԭ�Ϻϳ��Ҷ��ᣬ����NaBH4�����������·����ʻ���ԭ��Ӧ����CH3CH��OH��COOH������Ũ���ᡢ���������·�����ȥ��Ӧ����CH2=CHCOOH����������Ը�����������õ�HOOC-COOH���ϳ�·������ͼΪ��
��E�����ͬϵ��Ա�ͪ��Ϊԭ�Ϻϳ��Ҷ��ᣬ����NaBH4�����������·����ʻ���ԭ��Ӧ����CH3CH��OH��COOH������Ũ���ᡢ���������·�����ȥ��Ӧ����CH2=CHCOOH����������Ը�����������õ�HOOC-COOH���ϳ�·������ͼΪ�� ��
�� ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�����Ƶ���ˮ��������ɫ���ƿ�У������������� |
| B��ʵ�����ռ�NO��NO2ʱ��ֻ�����ſ����� |
| C���ռ���Һ���ڴ�ĥ�ڲ��������Լ�ƿ�� |
| D������Һ��ʱӦ��ˮ����Һ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���٢ۢ� | B���ڢݢ� |
| C���ܢݢ� | D���ۢޢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ϩ�ͱ�����ʹ��ˮ��ɫ���ʲ�������ˮ������ϩ�ͱ� |
| B��HNO3���뱽���ױ������͡���ά�ص��л������Ҫ��Ӧ������Ũ���������� |
| C���������Ƶ�������ͭ����Һһ���Լ����ܼ����Ҵ�����ȩ�����ᡢ��������Һ����Ҫʱ�ɼ��ȡ� |
| D����������KmnO4��Һ��������÷ֲ㣬�²�Һ��Ϊ��ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ��B2H6 |
| ��H2O2/OH- |
 ��RCHO+H2O
��RCHO+H2O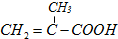 �������ķ�Ӧ��
�������ķ�Ӧ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��C3H8O��C2H6O |
| B��C2H4��C3H8 |
C�� ��C2H4 ��C2H4 |
| D��CH2O��C2H4O2 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com